The Aftermath of Hydrocodone Rescheduling: Intentional and Unintended Consequences
Pergolizzi JV1, Breve F2, Taylor R1, Zampogna G1,3, LeQuang JA1*
1 NEMA Research, Inc., Naples, Fla, USA.
2 Department of Pharmaceutical Sciences, Temple University School of Pharmacy, Philadelphia, Penn.
3 Department of Medicine, St. Vincent Charity Medical Center/Case Western Reserve University School of Medicine, Cleveland, Ohio.
*Corresponding Author
Jo Ann LeQuang,
NEMA Research, Inc., Naples, Fla, USA.
Tel: (979)864-4479/ (979)824-0251 (cell)
E-mail: joann@leqmedical.com
Received: December 19, 2016; Accepted: January 09, 2017; Published: January 11, 2017
Citation: Pergolizzi JV, Breve F, Taylor R, Zampogna G, LeQuang JA (2017) The Aftermath of Hydrocodone Rescheduling: Intentional and Unintended Consequences. Int J Anesth Res. 5(1), 377-382. doi: http://dx.doi.org/10.19070/2332-2780-1600078
Copyright: LeQuang JA© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The U.S. consumes about 99% of the world’s supply of hydrocodone, primarily in hydrocodone combination products (HCPs). The Drug Enforcement Administration’s (DEA) rescheduling of HCPs from Schedule III to the more restrictive Schedule II has changed prescribing patterns. The purpose of our article was to revisit HCP rescheduling to determine the impact this change had and its related consequences. Before 2014, DEA drug classification (scheduling) caused the “Vicodin loophole” which allowed HCP products to be prescribed under less restrictive conditions than single-entity hydrocodone products or oxycodone combination products. The rescheduling of HCPs to the more restrictive Schedule II has resulted in a decrease in HCP use but increased use of other analgesics. Unintended consequences of the rescheduling may include additional healthcare provider work, the potential for added costs, and patient inconvenience. For some patients, the rescheduling of HCPs may mean that they no longer have access to their preferred or effective analgesic or that they have been switched to another possibly less effective or tolerable analgesic. While the rescheduling has reduced the prescribing of hydrocodone, it is not apparent that it resulted in a net decrease in opioid use.
2.Methods
2.1. A Short History of Hydrocodone Combination Products
2.2. Prescribing Patterns
3.Discussion
4.Conclusion
5.Acknowledgements
6.References
Introduction
In 2014, when hydrocodone fixed-dose combination products (HCPs) were still Schedule III drugs on the Controlled Substances List [1], they were the most-prescribed drug in the United States [2]. Hydrocodone is an unusual opioid in that it is almost exclusively consumed in the U.S., which consumes about 99% of the world’s supply, primarily in fixed-dose combination products [3].
Clearly, the rescheduling of HCPs from Schedule III to the more restrictive Schedule II by the Drug Enforcement Administration (DEA) on August 22, 2014 was undertaken as a well-intentioned step to help stem opioid abuse in America. Just as selecting the appropriate analgesic product for a patient involves balancing medical benefits against risks, a regulatory decision to change drug scheduling should balance the benefits of such a change (reducing access to HCPs) against the possible unintended consequences of limiting legitimate access to pain relievers to the vulnerable population of pain patients.
HCPs occupy an important position on the analgesic continuum. The World Health Organization (WHO) states in its pain ladder that when a patient’s pain cannot be adequately controlled with nonopioid pain relievers, a weak opioid, such as codeine, may be recommended [4]. However, a substantial proportion of the Caucasian population are considered “poor metabolizers” and may not respond well or predictably to codeine therapy owing to certain genetic polymorphisms (nonfunctioning CYP2D6 alleles) or other factors [5]. For such patients, fixed-dose combination products offering a small amount of opioid combined with a nonopioid agent (such as acetaminophen) may be a preferable alternative to monotherapy with a stronger opioid, such as morphine or oxycodone.
HCPs combine two analgesic agents with complementary mechanisms of action (typically hydrocodone plus acetaminophen) that have been shown to be effective in multimechanistic pain syndromes [6, 7]. Such combination products are sometimes described as “opioid sparing” in that they rely on a relatively small amount of opioid [8].
The purpose of our article was to revisit hydrocodone rescheduling two years later and to determine the impact this change had upon prescribing practices and if there were consequences related to patients, opioid misuse, and pain control.
Methods
Although hydrocodone is also used in fixed-dose combination products such as an antitussive, our research was restricted to its role as an analgesic agent. We searched the literature using Pub-Med, Cochrane, and Embase databases for “hydrocodone rescheduling.” We included only articles that appeared since 2014 and which were available to us in English. We also examined the references of these articles and did general research on the history of the drug and these changes. Our primary interest was in retrospective studies and other analyses that reported on prescribing patterns or other effects following the rescheduling of hydrocodone.
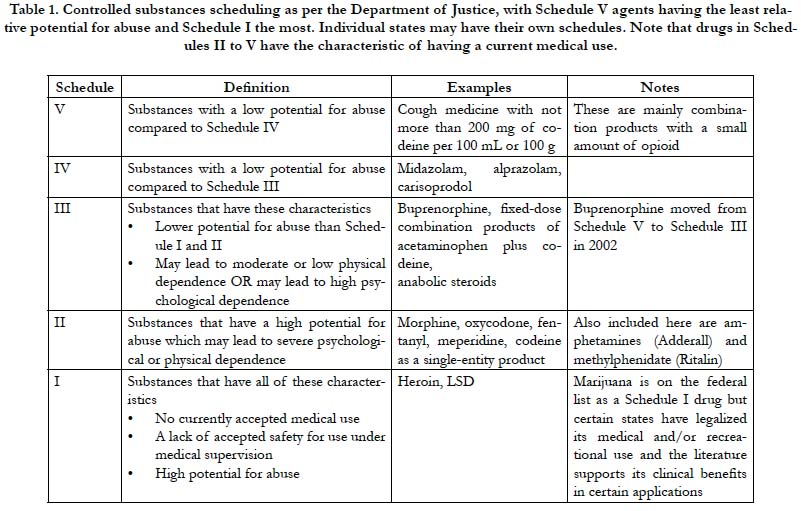
The Controlled Substances Act of 1970 categorizes drugs into five main classifications known as schedules [9]. Schedule I agents are the most and Schedule V the least restricted drugs. See Table 1. The Controlled Substances Act establishes federal drug scheduling; states are free to devise their own schedules, which cannot be less restrictive than the federal determinations. The administration and individual states sometimes reschedule drugs as knowledge of drug pharmacokinetics and pharmacodynamics increase or public health trends emerge. For example, in 2002, buprenorphine was moved from Schedule V (least restricted) to Schedule III [10]. HCPs were established as Schedule III drugs.
Single-entity oxycodone and fixed-dose combination products with oxycodone, such as oxycodone plus acetaminophen (Percocet ®, Endo Pharmaceuticals, Malvern PA) are both Schedule II drugs. Thus, restrictions are equivalent whether a prescriber selects the single-entity oxycodone product or the fixed-dose opioid-sparing product. The exception was hydrocodone. Fixeddose combination products with hydrocodone, such as hydrocodone plus acetaminophen (Vicodin®, Abbott Laboratories, North Chicago, IL) were classified as Schedule III agents while a new single-entity hydrocodone product was released to market in 2013 (Zohydro™ ER, Zogenix, Inc., San Diego, CA) as Schedule II [11]. This created what had been termed the “Vicodin loophole,” which allowed HCP products to be prescribed under less restrictive conditions than oxycodone combination product or the single- entity hydrocodone products [12]. In 2014, the DEA rescheduled HCPs as the more restrictive Schedule III, effectively closing the Vicodin loophole.
It should have come as no surprise that HCPs would be rescheduled to a more restrictive classification. The first petition by physicians suggesting this increased restriction was made in 1999 [13]. More recently, the DEA requested that the Department of Health and Human Services (HHS) provide a scientific and medical evaluation of HCPs along with a recommendation for scheduling. In December 2012, the FDA Public Advisory Committee published in the Federal Register that a public meeting regarding the proposal to reschedule HCPs would be held in January 2013. Starting in February 2013, stakeholders were invited to comment on the proposed rule, with published comments suggesting possible long-term and short-term ramifications of such a rescheduling [1].
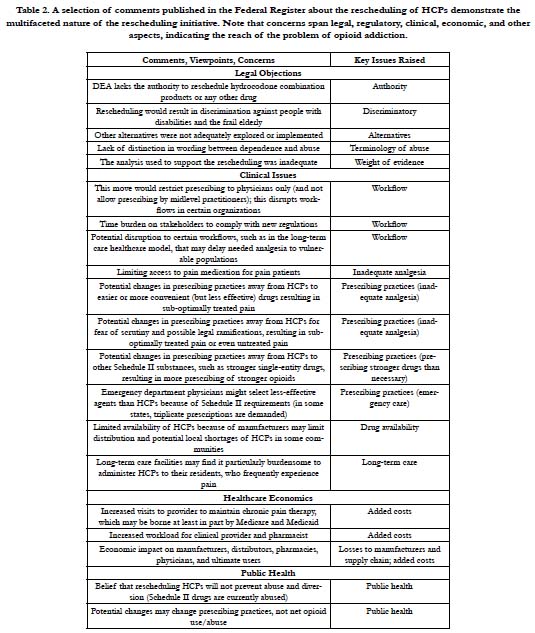
In the comment phase, individuals offered their own insights and personal accounts. Some shared their experiences with opioidaddicted family members and stated that HCPs were “gateway drugs.” Others supported the use of HCPs as important analgesic agents. Overall, 52% of those who made comments favored the decision to reschedule HCPs and make them more restricted (n=298) versus 41% who opposed this rescheduling and 7% who had no clear opinion. It should be noted that of the individuals who favored rescheduling HCPs to a more restrictive classification, 62% were members of the general public; physicians and pharmacists were less likely than the public to favor rescheduling [1]. Of the subsets of physicians and pharmacists who made comments, 44% and 60%, respectively, did not want HCPs rescheduled to a more restrictive classification [1]. The reasons given for opposing rescheduling HCPs were diverse. See Table 2.
In early 2013, an FDA advisory committee voted 19 to 10 to change the scheduling of HCPs from Schedule III to Schedule II, and in December 2013, HHS recommended that the DEA reschedule HCPs accordingly [13]. HCPs moved from Schedule III to Schedule II on October 6, 2014 and have remained there since that time.
It should be noted that in 2013 and 2014, two new single-entity extended-release (ER) hydrocodone products came to market in the U.S.: (Zohydro™ ER and Hysingla™ ER, respectively) [14]. These products were cleared for market as Schedule II substances.
A retrospective study of opioid prescriptions from July 2014 through January 2015 in Texas surveyed the time periods immediately before and after the rescheduling of HCPs [15]. The study evaluated the number of prescriptions for the most commonly prescribed HCPs (5 and 10 mg of hydrocodone combined with 325 mg of acetaminophen) along with Schedule III agents (tramadol, codeine/acetaminophen 30/300 mg and 60/300 mg). Morphine was also tracked as a control. After the rescheduling of HCPs, there was a 58% decrease in the number of prescriptions written for 5/325 mg hydrocodone/acetaminophen products and a 34% decrease in 10/325 mg products. This decrease in number of prescriptions was offset by increases for tramadol (up 17%) and codeine/acetaminophen (up 597% for 30/300 mg and up 1056% for 60/300 mg). During this time period, the number of prescriptions for morphine remained stable. A similar pattern emerged when evaluating the quantity of medications dispensed: HCPs decreased 42% (5/325 mg) and 14% (10/325 mg) while tramadol increased by 9%, and codeine 30/300 and 60/300 increased by 122% and 828% respectively. When the quantity of all opioid medications was evaluated, an overall net decrease of 6% was observed after HCPs were rescheduled-but if drugs were converted to morphine equivalents, the result was a 3% decrease [15].
A retrospective study of HCP prescribing patterns at a Level I trauma hospital system was conducted of 88,428 prescriptions before and after the rescheduling of hydrocodone in October 2014 [16]. The study also evaluated prescriptions for tramadol and analgesic products containing codeine. HCP prescriptions decreased from an average of 225.97 per day prior to rescheduling to 1.20 per day after rescheduling, while tramadol increased from 60.04 per day to 91.85 and codeine-containing products increased from 6.81 per day to 98.94 per day [16]. This suggests a similar pattern, namely that the rescheduling resulted in a decrease in HCP use but an increase in the use of other analgesics.
A retrospective study of the Texas Poison Centers before and after HCP rescheduling compared all prescription opioid exposures six months before and six months after rescheduling [17]. Hydrocodone exposures dropped significantly by 28% (from 1567 to 1135) after rescheduling (p=0.00017) for all ages, while codeine exposures increased significantly by 176% (from 189 to 522, p = 0.00014) including a 263% increase for the age group >20 years. Codeine misuse increased by 443% and adverse drug events associated with codeine increased by 327%. Oxycodone exposures increased by 39% (from 134 to 189, p = 0.0143) but only for patients over 20 years of age. Tramadol exposures increased significantly by 6% (from 666 to 708, p = 0.0193) and reported heroin exposures in the same time frame went up 15% but did not achieve statistical significance (from 156 to 179, p = 0.2286). This study suggested that rescheduling did indeed affect prescribing practices and resulted in decreased HCP prescribing but with a concomitant increase in the use of codeine, tramadol, and oxycodone. Heroin exposures were included in this study to determine if HCP rescheduling might have resulted in changes of drug street availability [17].
An online survey was conducted of 6,420 fibromyalgia patients to assess their reactions to the first 100 days after hydrocodone was rescheduled [18]. The majority of respondents (82.5%) had been prescribed a hydrocodone product for at least one year. Most respondents reported some barriers to accessing HCPs after the rescheduling. Some could no longer obtain HCPs and of this group, 18.1% borrowed pain medications from others and 17.1%, 13.1%, and 2.3% turned to other agents, namely marijuana, alcohol, or illicit drugs, respectively. The majority of respondents (64.2%) said that since the rescheduling of HCPs, they had to visit their healthcare providers more often. Of those fibromyalgia patients still working, 46.2% said these regulations resulted in their missing time from work. When asked if they thought thatrescheduling HCPs was an appropriate and fair way of dealing with the public health crisis of opioid abuse, 88.3% of these patients said no [18].
A retrospective study from the IMS Health National Prescription Audit, which reports on an estimated 80% of all dispensed retail prescriptions in the U.S., found that in the 36 months preceding hydrocodone rescheduling, the number of dispensed HCP prescriptions decreased by 8.4% and the number of dispensed tablets (by tablet count) decreased by 6.0% while overall the dispensed prescriptions and tablets for non-HCP opioids decreased by 0.2% and 0.5%, respectively [19]. Following hydrocodone rescheduling, HCP dispensing dropped sharply. HCP prescriptions decreased 22.0% in the first year after rescheduling compared to the 12 months preceding rescheduling and dispensed HCP tablets decreased by 16.0% in the same period. The main reason for the sharp decline in HCP product dispensing was the elimination of automatic refills; refills accounted for 73.7% of the drop; by March 2015, all refills were eliminated. In the same time period, non-HCP opioid analgesics increased by 4.9% (prescriptions) and 1.2% (tablet count) [19].
Discussion
There is no doubt that the rescheduling of hydrocodone was effective in terms of reducing the amount of HCP products prescribed and dispensed. In the first year after rescheduling, there were 26.3 million fewer HCP prescriptions written and 1.1 billion fewer HCP tablets dispensed compared to the year immediately prior to rescheduling [19]. The questions remain, however, as to whether or not this rescheduling merely shifted prescribing patterns or whether it resulted in a net decreased in opioid use. In the case of the latter, the possibility must be considered that a decrease in prescribed HCPs or prescribed opioids may be associated with unintended consequences.
The rescheduling of HCPs has not resulted in markedly less opioid prescribing but has simply changed prescribing patterns, namely fewer prescriptions are written for HCPs while more are written for other agents, such as codeine combination products and tramadol. Several retrospective studies have found that the rescheduling of hydrocodone did indeed result in a marked and often significant decrease in hydrocodone prescribing but it was matched by an increase in prescriptions for other drugs, in particular tramadol and products containing codeine.
Regulations associated with Schedule II substances compared to Schedule III substances mean that clinicians are likely to be more adversely affected by the rescheduling of HCPs than are drug addicts [20]. Additional administrative tasks now mean that prescribers will either have to accept the greater burden associated with Schedule II substances or find another, similar, Schedule III product. This may pose some unforeseen consequences. For example, codeine metabolism is variable among a subset of Caucasians known as “poor metabolizers” and another group known as “ultrarapid metabolizers.” This can not only affect the effectiveness of analgesia but it may also result in morbidity and mortality [21-24]. Indeed, the American Academy of Pediatrics has issued warnings in their guidelines about its concerns regarding the safety of codeine in children [25]. Hydrocodone is not subject to such a variable response. Tramadol is an effective analgesic but may also be subject to variability based on CYP2D6 polymorphisms [26]. Further, tramadol may be associated with serotonin syndrome when it is prescribed with certain other drugs, including many antidepressants [27]. Thus, the migration from one type of analgesic product to another is not necessarily helpful and certainly not without consequence. In particular, the scheduling change for HCPs is of particular concern if codeine-containing products become more widely used [25].
Chronic pain and opioid misuse may be described as dual epidemics. While it is easy to be caught up in the dramatic statistics about opioid misuse and abuse, the fact is that chronic pain is an equally urgent albeit more silent epidemic. An online survey of fibromyalgia patients--most of whom regularly took HCPs to manage their chronic painful condition--found 61% encountered some degree of difficulty getting their usual medications after hydrocodone rescheduling with the result that most had to visit their healthcare providers more frequently (and many missed work because of it), many who could no longer get HCPs were turning to illicit drugs or borrowing pain relievers from others, and over a quarter (27.2%) were in such despair over being denied ready access to HCPs that they had thoughts of suicide [18]. In this online survey (n = 6,420) patients received less effective drugs, had greater inconvenience, missed more work, and suffered greater despair when they were denied a standard pain control remedy that they had taken regularly up until the governmental rescheduling. Thus, the “benefit” of reducing HCP use is more than offset by the risk of patients turning to borrowed or illegal drugs.
In short, the change in scheduling for HCPs resulted in less HCP prescribing and consumption, but increases in other drugs. This in effect reduced prescribing choices for those physicians who wanted to offer a Schedule III rather than a Schedule II agent. When that restriction in choice results in greater prescribing of codeine-containing products, this migration is of questionable value, since genetic polymorphisms in a subset of Caucasian patients mean that response to codeine can be variable with potentially life-threatening consequences. Furthermore, the change in scheduling resulted in changes to clinical workflows and workloads in healthcare centers across the country and these changes can exacerbate the already overburdened environment of busy medical centers, hospitals, and long-term care facilities.
Conclusion
In an attempt to curtail the epidemic of opioid misuse, the DEA reclassified HCPs in August 2014 by moving them from Schedule III into the more restrictive Schedule II, but this action may have brought about unintended consequences. The rescheduling did indeed reduce the number of HCPs prescribed and the amount of HCPs consumed, but it increased the prescribing of other opioids, such that the result appears to be a migration in prescribing patterns away from HCPs and toward other products. Those prescribers wanting to prescribe Schedule III opioids might prescribe tramadol or codeine instead of HCPs, although these products may not be the best choice for their patients. Codeine, in particular, may be subject to variable response among patients owing to heritable conditions that lead to ultrarapid or poor metabolism. Patients previously prescribed HCPs may have been prescribed less effective or less tolerable products. Thus, two years after the rescheduling of HCPs, it appears that prescribing patterns rather than net use were disrupted.
Acknowledgements
The authors gratefully acknowledge the support of Robert B. Raffa, PhD, who was kind enough to read the manuscript in advance and provide helpful suggestions.
References
- Federal Register (2014) Schedules of Controlled Substances: Rescheduling of Hydrocodone Combination Products from Schedule III to Schedule II. 21 CFR Part 1308. .
- DEA. Hydrocodone, Drug Fact Sheet 2016.
- United Nations. Report on the International Narcotics Control Board for 2008, 2008.
- World Health Organization. WHO's pain ladder for adults, 1988.
- Smith H (2008) Variations in opioid responsiveness. Pain physician. 11(2): 237-248.
- Barkin RL (2001) Acetaminophen, aspirin, or Ibuprofen in combination analgesic products. Am j ther. 8(6): 433-442.
- Raffa RB, Tallarida RJ, Taylor R Jr., Pergolizzi JV Jr (2012) Fixed-dose combinations for emerging treatment of pain. Expert opin pharmacother.13(9): 1261-1270.
- Ahlbeck K (2011) Opioids: a two-faced Janus. Current medical research and opinion. 27(2): 439-448.
- FDA. Controlled Substances Act, 2009.
- Office of Diversion Control. Schedules of Controlled Substances: Rescheduling of Buprenorphine from Schedule V to Schedule III, 2002.
- Extended-release hydrocodone (Zohydro) for pain (2014) The Medical letter on drugs and therapeutics. 56(1444): 45-46.
- Goldberg D A (2014) A hydrocodone change with unknown side effects. Capital.
- Yap D. Hydrocone moved to Schedule II in DEA final rule, 2014.
- Covvey JR (2015) Recent developments toward the safer use of opioids, with a focus on hydrocodone. Res social admin pharm. 11(6): 901-908.
- Seago S, Hayek A, Pruszynski J, Newman MG (2016) Change in prescription habits after federal rescheduling of hydrocodone combination products. Proc (Bayl Uni Med Cent). 29(3): 268-270.
- Schultz S, Chamberlain C, Vulcan M, Rana H, Patel B, et al., (2016) Analgesic utilization before and after rescheduling of hydrocodone in a large academic level 1 trauma center. J opioid manag. 12(2): 119-122.
- Haynes A, Kleinschmidt K, Forrester MB, Young A (2016) Trends in analgesic exposures reported to Texas Poison Centers following increased regulation of hydrocodone. Clin toxicol (Phila). 54(5): 434-440.
- Chambers J, Gleason RM, Kirsh KL, Berner J, Fudin J, et al., (2015) An Online Survey of Patients' Experiences Since the Rescheduling of Hydrocodone: The First 100 Days. Pain med . 17(9): 1686-93.
- Jones CM, Lurie PG, Throckmorton DC (2016) Effect of US Drug Enforcement Administration's Rescheduling of Hydrocodone Combination Analgesic Products on Opioid Analgesic Prescribing. JAMA Intern Med. 176(3): 399-402.
- Pergolizzi JV Jr (2015) DEA Reschedules Hydrocodone Combination Products. Pain practice : the official journal of World Institute of Pain. 15(2): 95-97.
- Madadi P, Koren G, Cairns J, Chitayat D, Leeder JS, et al., (2007) Safety of codeine during breastfeeding: fatal morphine poisoning in the breastfed neonate of a mother prescribed codeine. Can fam physician. 53(1): 33-35.
- Madadi P, Ross CJ, Hayden MR, Carleton BC, Leeder JS, et al., (2009) Pharmacogenetics of neonatal opioid toxicity following maternal use of codeine during breastfeeding: a case-control study. Clin pharmacol ther. 85(1): 31-35.
- Madadi P, Shirazi F, Walter FG, Koren G (2008) Establishing causality of CNS depression in breastfed infants following maternal codeine use. Paediatric drugs. 10(6): 399-404.
- Sadhasivam S, Myer CM (2012) Preventing opioid-related deaths in children undergoing surgery. Pain medicine (Malden, Mass.). 13(7): 982-983.
- Fleming ML, Wanat MA (2014) To prescribe codeine or not to prescribe codeine? J pain palliat care pharmacother. 28(3): 251-254.
- Borlak J, Hermann R, Erb K, Thum T (2003) A rapid and simple CYP2D6 genotyping assay--case study with the analgetic tramadol. Metabolism: clinical and experimental. 52(11): 1439-1443.
- Park SH, Wackernah RC, Stimmel GL (2014) Serotonin syndrome: is it a reason to avoid the use of tramadol with antidepressants? J pharm pract. 27(1): 71-78.