Human Placentae in Pregnancy Induced Hypertension - A Histologic and Morphometric Analysis
Rohini P1, Siddharth T2, Fathima A3, Dhiren P4*
1 Assistant Professor, Department of Anatomy, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
2 Undergraduate Student, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
3 Postgraduate Student, Department of Anatomy, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
4 Associate Professor, Department of Physiology, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Karnataka, India.
*Corresponding Author
Dr. Dhiren Punja,
Associate Professor, Department of Physiology,
Kasturba Medical College, Manipal,
Manipal Academy of Higher Education, Karnataka, India.
Tel: +0820 2922327
E-mail: dhiren.punja@manipal.edu
Received: July 31, 2018; Accepted: August 24, 2018; Published: August 27, 2018
Citation: Rohini P, Siddharth T, Fathima A, Dhiren P. Human Placentae in Pregnancy Induced Hypertension - A Histologic and Morphometric Analysis. Int J Anat Appl Physiol. 2018;4(3):95-98. doi: dx.doi.org/10.19070/2572-7451-1800017
Copyright:Dhiren P©2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Introduction: Placenta is the mirror of maternal and fetal status. It has been called a pregnancy diary that permits the clinician to study the intra uterine environment of the fetus and some of the fetal responses to disease. Reduced maternal utero placental blood flow leading indirectly to constriction of foetal stem arteries has been associated with the changes seen in the placentae of pre-eclamptic women. Maternal vasospasm leads to fetal hypoxia, causing fetal distress and death. Histopathological changes related to confined placental mosaicism may be associated within adequate placentation and retroplacental ischemia.
Aim: To study morphometry and histological changes in placentae of women with pregnancy induced hypertension (PIH) and to observe the perinatal outcome of such pregnancies.
Materials: Total number of 50 placentae were used for the present study, out of which 25 placentae were obtained from women with PIH and 25 placentae from women who were normal having uncomplicated full-term deliveries.
Results: The mean weight of the placenta was 470.04g in control group and 385.70g in PIH group. Histopathological examination of the placentain PIH showed increase in number of syncytial knots and areas of fibrinoid necrosis.
Conclusion: PIH has remarkable influence on morphology and histology of the placenta, and there by affects the growth of fetus. The changes caused by placental insufficiency has an adverse affect on the neonatal birth weight due to compromised utero-placental blood flow.
2.Abbreviations
3.Introduction
4.Aim
5.Materials and Methods
5.1 Inclusion Criteria
5.2 Exclusion Criteria
6.Results
7.Discussion
8.Conclusions
9.References
Keywords
Feto-Placental Ratio; Perinatal Outcome; Vasospasm; Birth Weight; Histopathological.
Abbreviations
PIH: Pregnancy Induced Hypertension; H&E: Haematoxylin & Eosin; ISSHP: International Society for the Study of Hypertension in Pregnancy; DBP: Diastolic Blood Pressure; FP: Feto-Placental.
Introduction
The placenta is an organ that connects the developing foetus to the uterine wall to allow nutrient uptake, provide thermoregulation to the fetus, waste elimination, and gas exchange via the mother's blood supply, fight against internal infection and produce hormones to support pregnancy. Placenta is a vital organ for fetal development and is a mirror of maternal and fetal status. Complications in pregnancy like gestational diabetes or hypertension are reflected in the placenta in a conspicuous way [1]. According to WHO systematic review, hypertensive disorders of pregnancy contribute to 16% of maternal mortality, which is more than other leading causes namely hemorrhage (13%), abortion (8%) and sepsis (2%) [2]. Hypertensive disorders are also the cause of fetal and neonatal mortality and morbidity. Hypertensive disorders complicate about 5-10% of all pregnancies. Researchers suggest that there is reduction in surface area of placenta in pregnancy induced hypertension (PIH). These changes have been attributed to constriction of the fetal stem arteries due to decreased utero-placental blood flow [1].
The placenta develops from 2 sources - the chorion frondosum (fetal component) and the deciduas basalis (maternal component).
Placenta helps in exchange of gases, passage of nutrients and IgG antibodies from maternal to fetal side and passage of waste products from fetal to maternal side. It also produces hormones like human placental lactogen, human chorionic gonadotropin, estrogen, progesterone and relaxin. On gross examination, a normal full term placenta appears disc shaped. It weighs around 500-600g with a diameter of 15-20cm [3]. The placenta shows 2 surfaces-maternal surface with rough cobblest one appearance due to presence of 15-20 cotyledons separated by grooves occupied by incomplete placental septa and a fetal surface which is smooth and transparent asitis covered by amnion. The umbilical cord is normally attached to the center of fetal surface [3].
Aim
1. To study the morphometry and histological changes in placentae of women with pregnancy induced hypertension (PIH).
2. To observe the perinatal outcome of such pregnancies, compare the results with those of normal pregnancies.
Materials and Methods
The study was conducted on 50 placentae obtained from the Department of Obstetrics and Gynecology after attaining due consent from patients and approval from the Institutional Ethics Committee (IEC743/2016).
25 placentae were obtained from women diagnosed with PIH and 25 placentae from women who were having normal uncomplicated full-term deliveries.
Patients suffering from chronic hypertension, renal failure, cardiac problems, gestational diabetes mellitus and multiple pregnancy were excluded.
The collected placentae were squeezed to evacuate the blood and washed under running tap water to remove clots. The membranes were trimmed and the site of insertion of the umbilical cord was noted. The diameter, the weight and the central thickness of placenta was measured. The diameter of the placentae were calculated by taking the mean value of the shortest & longest diameter of placenta. The placental surface area was calculated using the formula 1.
A= πxd1xd2/4 where:
A = placental surface area d1 = minimum diameter (cm)
d2 = maximum diameter (cm)
The number of cotyledons were counted from maternal side. The feto-placental ratio was calculated after obtaining weight of the baby. Data analysis was done by using Statistical Package for Social Sciences (SPSS) 19 version, and P<0.05 was considered statistically significant.
Two tissue sections were taken from each placenta-one from the center and one from the edge. The tissue sample was fixed in formalin fluid, processed to prepare paraffin embedded blocks and 5-micron thick sections were cut. The slides are stained with Haematoxylin & Eosin (H&E) stains. The stained sections were observed and photographed using Olympus BX43 microscope.
Results
In the present study the placental weight (380.70gm), placental diameter (16.04cm) and placental surface area (201.43cm2) was comparatively less in the PIH group in comparison to the control group. It was also observed that the birth weight was less in the PIH group (Table 1). The site of attachment of the umbilical cord was observed to be eccentric both in case of the PIH placentae as well as the control group (Table 2).
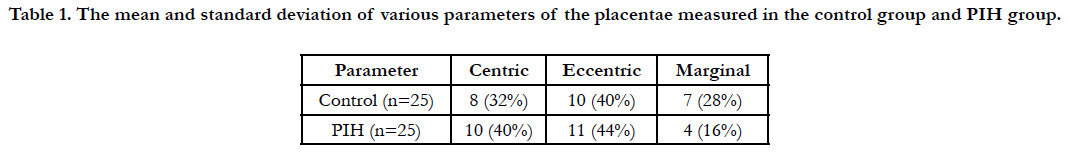
Histopathological examination of the placenta in PIH showed increase in number of syncytial knots, villous edema and areas of fibrinoid necrosis (Figure 1).
Discussion
Hypertensive disorders are seen in 5-10% of all pregnancies [2]. According to the International Society for the Study of Hypertension in Pregnancy (ISSHP), hypertension is defined as a diastolic blood pressure (DBP) > 90mm Hg or Systolic blood pressure (SBP) >140mm Hg on atleast 2 occasions taken 6 hours apart. In normal pregnancy, the DBP begins of all in early pregnancy and continues of all in second trimester or each anadirat 22-24 weeks. Then it steadily rises to reach prepregnant levels by term. This fall is due to reduced vascular tone, which leads to peripheral vasodilation. Pregnancy may aggravate hypertension in women, who are already hypertensive and may also induce hypertension in those who are nor motensive before they became pregnant. 3 grades of PIH are recognized: gestational hypertension, pre-eclampsia and eclampsia [1]. What ever the cause of hypertension, the potential complications are placental insufficiency, fetal growth restriction, preterm labour, abruption placentae, renal changes and maternal convulsions [2, 4].
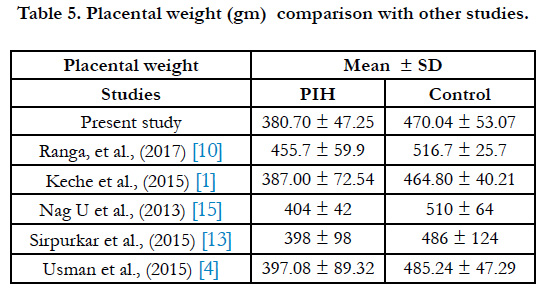
The mean weight of the placenta was 470.04gm in control group and 385.70g min PIH group (Table 5). According to Dutta and Dutta as grade of PIH increases, there is decrease in the weight of placenta [5]. Placental weight affects the fetal weight due to its relation with the villous surface area. Sirpurkar et al., observed that placental weight was quite low in PIH group than normal [13]. Placental weights show great variations which has been attributed to differences in genes regulating placental and fetal weight [6].
The mean placental diameter in control group and PIH group was 19.45 ± 6.53cm and 16.08 ± 4.63cm respectively (Table 1). In the study by Goswami et al., [7]. The values in control group were 21.225 ± 1.3585cm and 14.208 ± 1.2103cm in PIH group. Sultana et al., and Ashfaq et al., observed differences in placental weight and diameter between PIH group and controls, but these differences were not significant [8, 9]. The mean placental thickness was recorded as 1.96 cm and 1.57cm in control group and PIH group respectively. In preterm infants, thickness and weight of placenta vary directly with duration of pregnancy [1, 4].
The fetus derives its nutrition from the maternal blood. Fetal growth thus depends on the placental surface area through which diffusion of nutrients occurs from them aternal blood to the fetal circulation through the placental barrier. In PIH group, placental surface area was found to be 201.43 ± 42.61cm2 which was less than the mean placental surface area in the normal placentae (Table 1). However the difference was not found to be significant. In the present study, the number of cotyledons in the placentae from PIH group were found to be less than that of the control group (Table 1), similar to her studies [8, 10] however the difference between controls and cases was not found to be significant, similar to results obtained by Manjunath et al., and Mandhana et al., [11, 12].
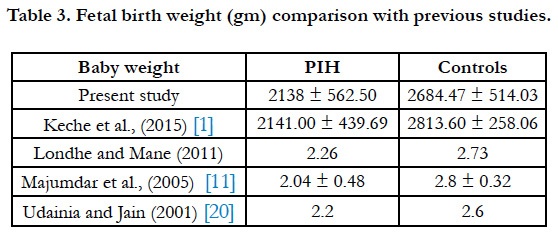
In our study the mean birth weight of babies was 2138 ± 438.50g min PIH group and 2684.47 ± 514.03g min control group (Table 3). According to one study placental weight and the placental deciduas area have a definite influence on the fetal birth weight [14]. Some studies have shown that size and weight of placenta is directly proportional to the birth weight of babies [1]. This may be due to altered intra cotyledonous vasculature seen in hypertension [15]. The site of attachment of umbilical cord did not show any significant differences between PIH group and control group in our study (Table 2).
Maternal vasospasm in PIH leads to decreased utero-placental blood flow. Fetal complications in PIH include intra uterine death, intra uterine growth retardation (due to chronic placental in sufficiency), asphyxia and prematurity. The fetal risk in PIH is related to severity of pre-eclampsia, duration of disease and degree of protein uria. Metabolic abnormalities such as impaired glycolytic pathway, poor utilization of glucose and decrease in oxygen consumption have been observed in placentae obtained from PIH pregnancies [1].
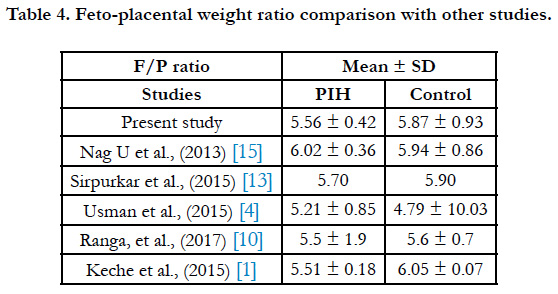
The feto-placental weight (F/P) ratio was 5.56 ± 0.42 and 5.87 ± 0.93 in PIH group and control group respectively (Table 4). Macpherson opined that F/P ratio can be used for evaluation of placental weight deviation [16]. According to some studies if fetus is small, the placenta will also show diminished growth a sitisa fetal organ [17].
Histopathological examination of the placenta in PIH showed increase in number of syncytial knots and areas of fibrinoid necrosis. Areas of calcification and hyalinized villi were observed along with a cute atherosis of decidual arteries. According to Foxetal, reduced utero-placental blood flow causes decrease in villous perfusion leading to excess syncytial knot formation and stromal fibrosis [18].
Studies have reported that hyalinization, endothelial proliferation of arteries and fibrinoid necrosis illustrate placental mosaicism and presumably the after math of PIH [19, 20]. Plausibly this may cause placental insufficiency leading to fetal complications encounter redin PIH.
Conclusions
The high incidence of pregnancy induced hypertension and its adverse effects on the placental morphology which directly affects the growth and nutrition of fetus in uterois of significant concern. Among the other morbidities of pregnancy such as hemorrhage and infection; PIH plays a crucial role. The complications as seen in this study not only affect macroscopically but also microscopically.
References
- Keche HA, Keche AS. Morphometric differentiation between placenta in PIH and normal pregnancy. Int J Med Sci Public Health. 2015 Feb 1;4:250- 5.
- Daftary SN, Chakravarti S, Pai MV, Kushtagi P. Holland & Brews Manual of Obstetrics. 4th ed. Reed Elsevier India Pvt. Ltd; 2016.
- Sing V. Textbook of Clinical Embryology. 1st ed. India: Elsevier; 2013.
- Usman JD, Bello A, Musa MA, Abdulhameed A, Bello SS, Ammani T, Zagga AD. Morphometric Assessment of the Placenta in Women with Gestational Hypertension: Findings from a Rural Community in Sokoto, Nigeria. Int J Health Sci Res. 2015;5(3):70-4.
- Dutta DK, Dutta B. Study of human placentae associated with preeclampsia and essential hypertension in relation to foetal outcome. J Obstet Gynecol India. 1989;39(6):757-63.
- Kher AV, Zawar MP. Study of placental pathology in toxaemia of pregnancy and its fetal implications. Indian J Pathol Microbiol. 1981 Oct;24(4):245- 51. PubMed PMID: 7338399.
- Goswami P, Memon S, Pardeep K. Histological and radiological study of calcified placenta. IOSR J Dent Med Sci. 2013;7(4):37-41.
- Sultana S, Hossain GA, Rahman MH, Hasan N, Sultana SZ, Khalil M. Changes of placental diameter thickness and cotyledon in eclampsia. Mymensingh Med J. 2007 Jul;16(2):127-31. PubMed PMID: 17703146.
- Ashfaq M, Janjua MZ, Channa MA. Effect of gestational diabetes and maternal hypertension on gross morphology of placenta. J Ayub Med Coll Abbottabad. 2005 Jan-Mar;17(1):44-7. PubMed PMID: 15929527.
- Siva SreeRanga MK, Adaline Thangam TF, MC Vasantha Mallika, MV Indira. Morphological and histological variations of human placenta in hypertensive disorders of pregnancy. Int J Anat Res. 2017;5(1):3591-3598.
- Majumdar S, Dasgupta H, Bhattacharya K, Bhattacharya A. A study of placenta in normal and hypertensive pregnancies. J Anat Soc India. 2005;54(2):1-9.
- Mandhana VS, Shroff GA, Kharkar AR, Nawal A. A Comparative Morphological Study of Placenta in Normal Pregnancy and Placenta of Pregnancy Induced Hypertension. Int J Health Sci Res. 2014;4(12):184-8.
- Sirpurkar M, Anjankar VP. Study of correlation between placental morphology and adverse perinatal outcome in different conditions affecting pregnancy. Int J Reprod Contracept Obstet Gynecol. 2017 Feb 9;4(4):1165-8.
- Younoszai MK, Haworth JC. Placental dimensions and relations in preterm, term, and growth-retarded infants. Am J Obstet Gynecol. 1969 Jan 15;103(2):265-71. PubMed PMID: 5764932.
- Nag U, Chakravarthy VK, Rao DR. Morphological changes in placenta of hypertensive pregnant women. Int J Res Rep Med Sci. 2013 Apr;3(2):1-4.
- Macpherson T. Fact and fancy. What can we really tell from the placenta?. Arch Pathol Lab Med. 1991 Jul;115(7):672-81. PubMed PMID: 2064525.
- Sawant LD, Venkat S. Comparative analysis of normal versus fetal growth restriction in pregnancy: The significance of maternal body mass index, nutritional status, anemia, and ultrasonography screening. Int J Reprod Med. 2013;2013:671954. doi: 10.1155/2013/671954. PubMed PMID: 25763389.
- Fox H. The morphological basis of placental insufficiency. J Obstet Gynaecol India. 1975 Aug;25:441-50.
- Teasdale F. Gestational changes in the functional structure of the human placenta in relation to fetal growth: a morphometric study. Am J Obstet Gynecol. 1980 Jul 1;137(5):560-8. PubMed PMID: 7386550.
- Udainia A, Jain ML. Morphological study of placenta in pregnancy induced hypertension with its clinical relevance. J Anat Soc India. 2001;50(1):24-7.