Thin Films of TiO2: Cu:Ag Cell Cytotoxicity and Antibacterial Activity
Stoyanova D1, Nesheva A1, Veleva R1, Borisova M1, Angelov O3, Ivanova I1, Kostadinova A2*
1 Faculty of Biology, Sofia University, 8 Dragan Tzankov Blvd, 1164 Sofia, Bulgaria.
2 Institute of Biophysics and Biomedical Engineering, Bulg. Acad. Sci., Acad. G. Bonchev str., Sofia, Bulgaria.
3 Central Laboratory of Solar Energy and New Energy Sources, Bulgarian Academy of Sciences, Tsarigradsko Chaussee, Blvd., Sofia, Bulgaria.
*Corresponding Author
Dr. Kostadinova A,
Institute of biophysics and biomedical engineering-BAS,
Faculty of Biology, 1113 Sofia, Bulgaria.
Tel: +35929792629/+359897946452
Email: aneliakk@yahoo.com
Received: December 12, 2017; Accepted: January 09, 2018; Published: January 11, 2018
Citation: Stoyanova D, Nesheva A, Veleva R, Borisova M, Angelov O, Ivanova I, Kostadinova A. Thin films of TiO2: Cu:Ag Cell Cytotoxicity and Antibacterial Activity. Int J Bioinform Biol Syst. 2018;2(1):25-29.
Copyright: Kostadinova A© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Self-cleaning surfaces are of increasing interest in various studies around the world. Static metal oxide layers are attractive for application to the walls of neonatal incubators, in sterile rooms, on dressings, and antimicrobial layers on catheters and implants. The aim of this study is to investigate the antibacterial and cytotoxicity effect of TiO2: Ag: Cu thin films. The thin films of TiO2: Ag: Cu are deposited on glass substrates without heating during the deposition by RF magnetron cosputtering of TiO2 target and pieces of Ag (60cmm2) and Cu (100mm2). The studied films, thickness about 60 nm, were deposited in argon atmosphere. Films tested by diffusion assay showed antibacterial effect to Е. coli, Salmonella entherica, Staphylococcus epidermidis and Bacillus cereus. The growth curve reveal the full destroying of S. epidermidis and Bacillus cereus cells. Crystal violet staining and phase-contrast microscopy observation were used to determine changes in the morphology and cytotoxicity of the epithelial cell line (A549). Our investigations show that these thin films of TiO2:Ag:Cu decrease the cell viability of the tested cultured lines and has good antibacterial effect against Gram - positive and Gram-negative bacteria. This material can be used for the medical application such as catheters, clean surfaces etc.
2.Introduction
3.Materials and Methods
3.1 Preparation of Thin Film Coated Glass Substrates
3.2 Antimicrobial Test
3.3 Crystal Violet Staining
4.Results
4.1.Cytotoxicity
5.Discussion
6.Acknowledgements
7.References
KeyWords
Thin Films; CuO; TiO2; Ag; Antibacterial Activity; Cell Cytotoxicity; Biomedical Application.
Introduction
Magnetron sputtering (rf Magnetron sputtering) is one of the most promising methods for nanostructured materials, due to the possibility of obtaining homogeneous coating over a large area at low temperatures and easily controlled technological conditions for synthesis. This is a physical method for depositing thin films by atomizing material obtained from bombardment with argon ions of a matrix of a given composition. Sprayed atoms are deposited on a substrate, such as a glass plate. Target atoms have different kinetic energy with which they leave the target. Among them there are ions which, depending on the conditions of the electric discharge, can reach the substrates and influence the structure of the forming layer. The sputtered gas used is generally an inert gas - argon (Ar). The presence of various parameters controlling the deposition makes the process complicated, but also allows a high degree of control over the growth and microstructure of the film [1, 2].
Various factors such as certain bacterial strains or bacterial proteins with enzymatic activity (e.g., protease, hyaluronidase, neuraminidase, elastase, and collagenase) can facilitate the invasion of microbial infections when there are defects in defence mechanisms or low resistance to microbial agents. Numerous microorganisms have mechanisms that impair antibody production by inducing suppressor materials. More than that, the adherence of microbial molecules to the surface plays a role in the development of microbial strains. The immediate immune response to bacterial infection is affected by a defect in the phagocytic system and can produce the development of severe pneumonias or recurrent abscesses. The binding of certain Gram-positive organisms (e.g., staphylococci) is favoured by host receptors such as cell surface proteins or cell surface sugar residues. Bacteria such as Escherichia coli and S enterica possess distinctive adhesive organelles called fimbriae or pili that allow them to attach to almost all human cells including neutrophils and epithelial cells in the genitourinary tract, mouth, and intestine. On the other hand, S. aureus is a type of bacteria commonly found on hair, skin, noses, and throats of people and animals and it multiplies rapidly at room temperature. S. aureus can cause serious food poisoning [3, 4]. Moreover, it is one of the most common causes of infections in the hospitals and may cause diseases. For this we use this bacteria in our investigation like model to discover how co-deposited TiO2, Ag, Cu on thin films affect cells.
The thin films were deposited on glass substrates by r.f. magnetron co-sputtering (13.56 MHz; r.f. power of 50W; in Ar atmosphere at 0.8 Pa) of TiO2 target with Ag and Cu pieces on the surface in its maximum erosion zone without heating during the deposition. The total surface area of Ag (4 alike pieces) was 60mm2 and this one of Cu (4 alike pieces) was 100 mm2. The size of the substrates was 20x25 mm, the glass was preliminary treated by solution of H2SO4: H2O2 (1:1) to ensure the adhesion of coating. The thickness of the films was about 60 nm, measured by profilometer Taylor Hobson.
Bacterial strains: In this study, we used the following bacteria: E. Coli ATCC10536, Bacillus cereus, Staphylococcus epidermidis ATCC 12228 all supplied by the Bulgarian National Bank for Industrial Microorganisms and Cell Cultures (NBIMCC).
Difussion assay: Diffussion assay was used for fast screening. The test bacterial strains were grown in the most suitable for each species growth medium at 180 rpm for 18h. The microbial density of exponential bacterial culture of 0.5 - 0.8 was determined according to McFarland. The aliquots of 100 μL microbial suspension were randomly spread on a solid nutrient medium (Conda, Spain), and the thin films of the investigated material were put on them. The plates were left for 20 h at 4 - 6°C to afford diffusion of the nanoparticles and after that were cultivated for 24 h at 37°C for all bacteria. The formed sterile zones around the samples were measured in mm (+ 0.5).
Disinfection time: Determination of disinfection time was conducted for 24 h in a light-dark regime with illumination for 15 min during the sampling in Corning® Costar® TC-Treated six-well plates. Two wells were used for control bare glasses (without thin films) and the other four wells thin film coated glass substrates were placed and sterilized by 30 min UV-irradiation. In the well with blank control, a sterile nutrient liquid medium was added without bacteria to measure the thin film dissolution and light absorption of the liquid (Nutrient broth, Conda). In the remaining 5 wells, liquid culture medium inoculated with a test microorganism with initial concentration (OD 0.001) of overnight bacterial culture in exponential phase was added. The inoculum and medium quantity in all variants were the same for all samples and control at the beginning of the experiment. The optical density was measured every hour with spectrophotometer Zeiss at λ = 610nm. Also, the determination of survived bacteria was studied through classical Koch's method. In every 2 h, consecutive decimal dilutions were prepared for determination of the bacterial amount of the samples and the controls. After plating of 100 μL bacterial suspension on the solid nutrient medium without thin films and cultivation at 37°C for all other bacteria and dark condition, counting of the bacterial colonies was conducted.
Cells: A549 lung alveolar epithelial cell line Company: ATCC Catalog: CCL-185 were grown in Dulbecco’s minimal essential medium (DMEM) containing 10% fetal bovine serum (Sigma) in a humidified incubator with 5% CO2. For the experiments, the cells were harvested from nearly confluent cultures with 0.05% trypsin/0.6 mM EDTA (Sigma).
Crystal violet staining was performed with some modifications as follows: the residual cell monolayer was washed with phosphatebuffered saline (PBS) and fixed with 4 % imperial gallon (ig) paraformaldehyde in PBS for 15 min. After that, plates were washed with water and 200 μl of 1 % ig crystal violet solutions was added to every well. After 20 min of incubation at room temperature, plates were washed with water and the protein-bound dye (which is corresponding to a cell number) was solubilized with 200 μl of 10 % ig acetic acid. The values of optical density were read on a microplate reader (Epoch UV/Vis spectrometer) at 570 nm wavelength.
Microscope pictures were taken under an invert microscope (XDS-2A, China) supplied with a digital camera (DV-130, China).
Results
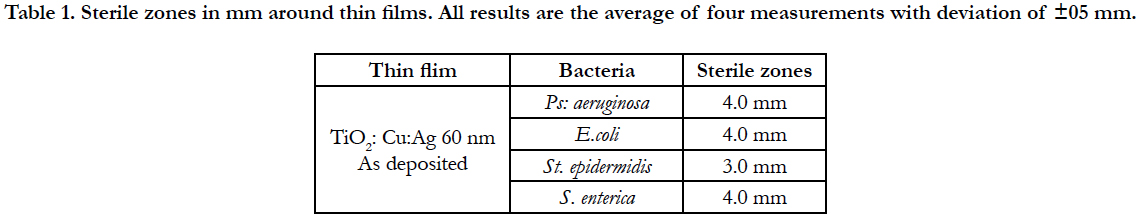
On Table 1 we demonstrate antibacterial effect of thin films. The amount of copper and silver added in the tested films is essential as our previous investigations have shown no antibacterial effect of TiO2 thin film and insignificant of the TiO2:Ag. The sensitivity of the tested bacteria to TiO2: Ag: Cu films of 60 nm SAg = 60 mm2, SCu = 100 mm2 is similar. The toxicity of the materials to the bacteria is dependent on the surface structure and composition of the bacterial cell wall.
The size of the sterile zones against the Gram-positive S.epidermidis bacteria is smaller, indicating the higher resistance of these bacteria to the investigated thin films. In Gracilicutes bacteria were measured bigger sterile zones, which could be explained with the thinner peptide-glucan layer in the cell wall of these bacteria.
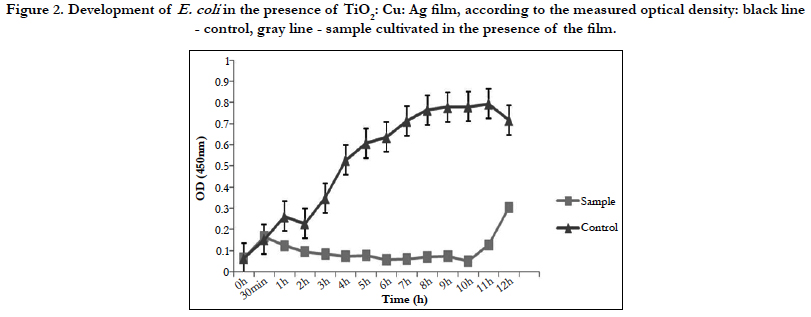
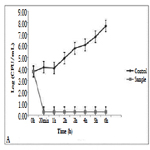
Figure 2 shows only bacteriostatic effect on E.coli and fast development of bacteria after the 10th h of the experiment.
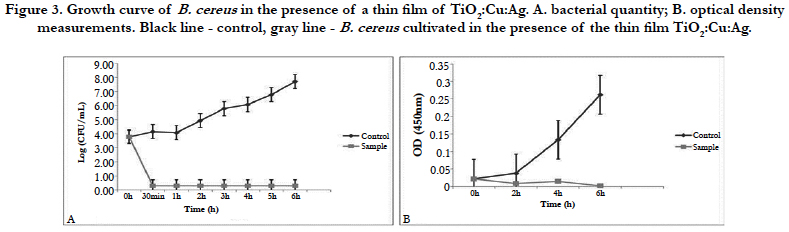
The TiO2:Cu:Ag thin film has a significant toxic effect on S. epidermidis (Figure 1). Progressive extinction of cells is observed up to 8th hours. This film has a 100% bactericidal effect on S. epidermidis since no bacterial growth was detected within 24 hours at 37°C.
Thin coatings of metals and metal oxides have a great potential in medical practice and lifestyle. From our research and the results obtained, it can be concluded that some of the nanostructured films have a significant bactericidal effect and would be useful in the future.
The effect of thin films depends on the composition, the mode of treatment (whether heat-treated or not) and the micro-organism tested. Our previous investigations have shown that annealed at 500°C thin films with the same composition have no antibacterial effect (results are not presented).
Thin films composed of TiO2: Ag: Cu have a pronounced bactericidal effect against S. epidermidis and B. cereus which reaches 100% after 24h of the experiment. The same result was confirmed by the dehydrogenase activity measured at the 24th hour of the experiment.
Against E.coli this film exhibits only bacteriostatic effect. Despite the drastic drop in the bacterial amount in the medium for the period of 4 hours, it is possible that a few resistant cells remain unaffected by the action of the thin film. The fact that most cells die is also confirmed by the measurement of dehydrogenase activity of E. coli at the 4th hour of the experiment. The lack of such activity confirmed our assumptions that only single resistant cells remained and the method is not enough sensitive to give clear evidence of their presence. Although titanium dioxide, copper, and silver films show a mixed bactericidal and bacteriostatic effect on different bacteria, we can conclude that the film has a strong enough effect to find real application in the medicine.
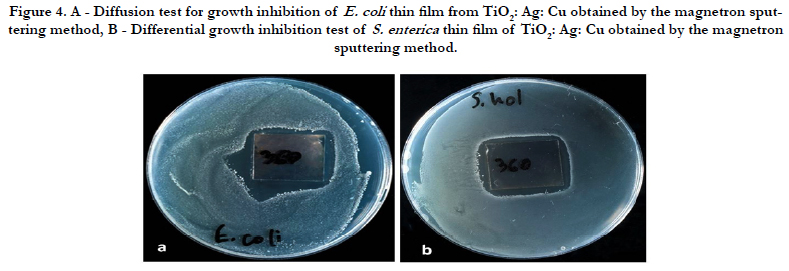
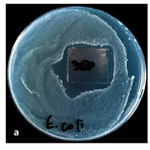
The presence of resistant cells E. coli was also observed in the agar diffusion experiments. This result can be seen in Figure 4. Despite the bright areas of enlightenment, a small number of single persistent colonies are observed in the vicinity of the film. The mechanisms of resistance are still unclear.
Cytotoxicity effect of TiO2: Ag: Cu thin films was tested toward model A549 epithelial cells.
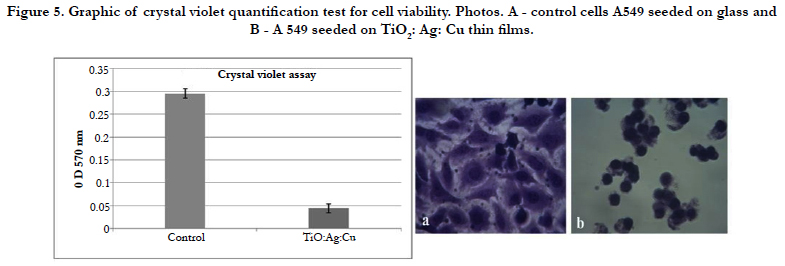
Crystal violet staining and phase-contrast microscopy observation for epithelial cell line A549 was used to determine changes in morphology and cytotoxicity. The A549 cells in control were very good attached and spread. Morphology is polygonal - typical for normal differentiated epithelial cells (Figure 5a). On the TiO2: Ag: Cu thin films A549 epithelial cells were unattached and roundshaped (Figure 5b) that is typical for the stressed or dying cells.
It is evident that the optical density values and, respectively, the protein-bonded crystal violet for all eukaryotic cells at TiO2: Ag: Cu thin films have shown a lower cells amount in the sample in contrast to the control cells (Figure 5 graphic). The survived quantity of cells on thin films was significantly under 30 %, indicating the high toxicity of the studied TiO2: Ag: Cu thin films.
The investigated TiO2: Ag: Cu thin films were cytotoxic for epithelial cells and in the same time possess also good antibacterial activity. This finding is very interesting because this thin films can be used in medical application where no need of attached eukaryotic cells is and in the same time material must be antibacterial. This material can be used in catheters, to the walls of neonatal incubators, etc, as was written above.
Discussion
Studies performed by Ciobanu et al., [5] on silver doped hydroxyapatite nanoparticles have proven a good antimicrobial activity for all the studied concentrations of silver [5] in the samples. Static layers have two types of antibacterial and cytotoxyc effect: a mechanical bactericidal effect consisting of attaching bacteria to the surface of the layer and mechanically breaking of the cell wall, and forming reactive oxygen species (ROS) on the surface of the layer that have a strong toxic effect on the bacteria. The toxic species formed are hydrogen peroxide (H2O2), superoxide anion O2-, peroxide O2-2, hydroxyl radical OH, hydroxyl ion OH-. The mechanical bactericidal effect is due to different physical phenomena as the particular properties of surface structure, regardless of its biochemical functional properties [6].
Not surprisingly, nanomaterials are attracting extensive interest in environmental science, where applications and implications [7, 8] of nanosheets are being studied. The ecotoxicology and environmental impact of thin films strongly depend on interactions with the membrane of living cells (like A 549 epithelial cell line), which serves as a barrier between intracellular contents and the surrounding environment [8]. Several mechanisms for interaction with cell membranes have been proposed for thin films nanomaterials, including chemical oxidation and physical disruption. Chemical oxidation can occur either through the generation of reactive oxygen species or through direct electron transfer [1]. Physical disruption may be initiated via direct contact with thin films, followed by penetration of the cell membrane [7, 8]. Loss of membrane integrity or extraction of lipid molecules may then generate through pore formation, adsorption or adhesion to the nanomaterial surface. This is the reason that our epithelial cells cannot attached on TiO:Cu;Ag thin films [9, 10]. This membrane disruption process depends strongly on the availability and orientation of sharp edges as well as the mechanical, morphological, and surface chemical properties of the nanomaterials [11]. However, selected mechanistic studies showed that Cu(II) and Ag(I) and its metal complexes inhibited DNA synthesis in eukaryotic cells which did not appear to be mediated through intercalation [12]. This means that Cu(II) and Ag(I) and its metal complexes can affect on eukaryotic cells and is one of the reasons for the cell apoptosis. It is not surprising that in our investigation epithelial cells cannot attach and die.
With the RF Magnetron sputtering method for thin films deposition we can modulate the thickness of the film and the quantity of metal ions in it. So we hope to prepare antibacterial film with low cytotoxicity for eukaryotic cells and high antibacterial activity to use it in medical application as wound dressing, catheters, dental implants etc.
Acknowledgments
The authors are thankful to prof. T. Vladkova for the collaboration. The present work was supported by the grand number. No 80-10-12 oт 12.04.2017- Science Fund – Sofia University ‘St. Kliment Ohridski’, DN 09-11/16.12.2016 NSF and COST Action TD1305 “Improved Protection of Medical Devices against Infections”.
References
- Dimova-Malinovska D, Angelov O, Nichev H, Pivin JC. ZnO: H thin films for room temperature selective NH3 sensors. In14th International School on Condensed Matter Physics 2006 Sep 17 (Vol. 9, pp. 248-251). INFM; INOE.
- Wang H, Wingett D, Engelhard MH, Feris K, Reddy KM, Turner P, Layne J, Hanley C, Bell J, Tenne D, Wang C. Fluorescent dye encapsulated ZnO particles with cell-specific toxicity for potential use in biomedical applications. Journal of Materials Science: Materials in Medicine. 2009 Jan 1;20(1):11.
- Saviuc C, Grumezescu AM, Holban A, Bleotu C, Chifiriuc C, Balaure P, Lazar V. Phenotypical studies of raw and nanosystem embedded Eugenia carryophyllata buds essential oil antibacterial activity on Pseudomonas aeruginosa and Staphylococcus aureus strains. Biointerface Res Appl Chem. 2011;1(3):111-8.
- Crisley FD, Angelotti R, Foter MJ. Multiplication of Staphylococcus aureus in synthetic cream fillings and pies. Public Health Rep. 1964 May; 79(5): 369–376. PubMed Central PMCID: PMC1915433.
- Ciobanu CS, Iconaru SL, Chifiriuc MC, Costescu A, Le Coustumer P, Predoi D. Synthesis and antimicrobial activity of silver-doped hydroxyapatite nanoparticles. Biomed Res Int. 2013; 2013: 916218. PubMed Central PMCID: PMC3591194.
- Li Y, Yuan H, von dem Bussche A, Creighton M, Hurt RH, Kane AB, Gao H. Graphene microsheets enter cells through spontaneous membrane penetration at edge asperities and corner sites. Proc Natl Acad Sci U S A. 2013 Jul 23; 110(30): 12295–12300. PubMed Central PMCID: PMC3725082.
- Tu Y, Lv M, Xiu P, Huynh T, Zhang M, Castelli M, et al. Destructive extraction of phospholipids from Escherichia coli membranes by graphene nanosheets. Nature nanotechnology. 2013 Aug 1;8(8):594-601.
- Moghadam BY, Hou WC, Corredor C, Westerhoff P, Posner JD. Role of nanoparticle surface functionality in the disruption of model cell membranes. Langmuir. 2012 Nov 27; 28(47): 16318–16326. PubMed Central PMCID: PMC3508167.
- Liu X, Chen KL. Interactions of graphene oxide with model cell membranes: probing nanoparticle attachment and lipid bilayer disruption. Langmuir. 2015 Oct 26;31(44):12076-86.
- Perreault F, De Faria AF, Elimelech M. Environmental applications of graphene- based nanomaterials. Chemical Society Reviews. 2015;44(16):5861- 96.
- Gilbertson LM, Albalghiti EM, Fishman ZS, Perreault F, Corredor C, Posner JD, Elimelech M, Pfefferle LD, Zimmerman JB. Shape-dependent surface reactivity and antimicrobial activity of nano-cupric oxide. Environmental science & technology. 2016 Mar 17;50(7):3975-84.
- Deegan C, Coyle B, McCann M, Devereux M, Egan DA. In vitro antitumour effect of 1, 10-phenanthroline-5, 6-dione (phendione),[Cu (phendione) 3](ClO 4) 2•4H 2 O and [Ag (phendione) 2] ClO4 using human epithelial cell lines. Chemico-biological interactions. 2006 Dec 1;164(1):115-25.