Multidetector Computed Tomography Features of Plasmablastic Lymphoma of the Oral Cavity in an HIV-Positive Patient
Almahndr MJ1, Barghan S2, Kashtwari D2, Tahmasbi Arashlow M3, Nair MK4*
1 Division of Radiology, Department of Oral and Maxillofacial Diagnostic Sciences, College of Dentistry, University of Florida, Center Dr, Gainesville, FL, USA.
2 Clinical Assistant Professor, Division of Radiology, Department of Oral and Maxillofacial Diagnostic Sciences, College of Dentistry, University of Florida, Center Dr, Gainesville, FL, USA.
3 Adjunct Assistant Professor, Division of Radiology and the Imaging Center, Department of Diagnostic Sciences, Texas A&M University College of Dentistry, Gaston Ave, Dallas, Texas, USA.
4 Professor and Director, Division of Radiology and the Imaging Center, Department of Diagnostic Sciences, Texas A&M University College of Dentistry, Gaston Ave, Dallas, Texas, USA.
*Corresponding Author
Madhu K. Nair,
Division of Radiology, Department of Oral and Maxillofacial Diagnostic Sciences,
Texas A&M University College of Dentistry, 3302 Gaston Ave, Dallas, Texas 75246, USA.
Tel: (214) 828-8393
Fax: (214)874-4557
E-mail: mknair@tamhsc.edu
Received: February 05, 2018; Accepted: February 20, 2018; Published: February 21, 2018
Citation:Almahndr MJ, Barghan S, Kashtwari D, Tahmasbi Arashlow M, Nair MK. Multidetector Computed Tomography Features of Plasmablastic Lymphoma of the Oral Cavity
in an HIV-Positive Patient. Int J Dentistry Oral Sci. 2018;5(2):601-605. doi: dx.doi.org/10.19070/2377-8075-18000117
Copyright: Nair MK©2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Overview: Plasmablastic lymphoma (PBL) is an uncommon aggressive malignancy that is associated with patients with human immunodeficiency virus (HIV), other immunosuppression conditions, or in elderly patients, that tends to appear in the oral cavity. PBL accounts for 2.6% of all HIV-associated non-Hodgkin lymphomas.
Case Report: We describe the case of a chemotherapy-treated patient with HIV-associated plasmablastic lymphoma who presented with a three-month history of left facial swelling. Features seen on different imaging modalities including panoramic and multi-detector computed tomography (MDCT) were suggestive of a malignancy and/or an inflammatory lesion. However, histopathological examination revealed an aggressive immunoblastic neoplasm, consistent with AIDS-associated PBL.
Conclusion: PBL is hard to diagnose, rapidly progressive, with overall poor prognosis among HIV-infected patients, and often rapidly fatal. The maxillofacial radiologist must be aware of the existence of this relatively newly described lymphoma as it is associated with immunosuppression and may be misdiagnosed as other pathoses.
2.Introduction
3.Case Report
4.Discussion
5.Conclusion
6.Acknowledgement
8.References
Keywords
Plasmablastic Lymphoma; PBL; HIV; AIDS; Multidetector Computed Tomography.
Introduction
Lymphoma is defined as a group of tumors that develop from lymph nodes [1]. Patients with lymphomas may experience fever, weight loss, night sweats, lymphadenopathy, and fatigue [1]. Hodgkin lymphomas (HL) and non-Hodgkin lymphomas (NHL) are the two primary kinds of lymphoma [2]. Plasmablastic lymphoma (PBL) is classified as a distinct entity of B-cell lymphoma in the recent WHO classification of lymphoid neoplasms [2]. Other names of this variety of lymphoma include anaplastic lymphoma kinase-positive diffuse large B-cell lymphoma (ALKDLBCL), plasmablastic plasmacytoma, and the human herpesvirus 8 (HHV8) - related primary effusion lymphoma (PEL) [2].
PBL is considered a rare lymphoma that may present in the oral cavity [3]. It was first noted by Delecluse et al., in 1997 and is known to have a strong association with human immunodeficiency virus (HIV) and Epstein-Barr virus (EBV) infections [4, 5]. Tyrosine kinases also displayed a pathogenic role in lymphomas in which anaplastic lymphoma kinase (ALK) is the most transforming gene of the majority of anaplastic large cell lymphomas (ALCL) [6].
The estimated frequency of PBL is about 2.6% of NHL cases with acquired immune deficiency syndrome (AIDS) [7]. 92% of oral PBL cases depicted in the English medical literature were HIV-positive patients [8]. However, PBL is also known to affect HIV-negative patients [9]. This case report describes the Multi- Detector Computed Tomography (MDCT) imaging features of oral PBL in an HIV-positive patient and discusses the progression of this condition.
Case Report
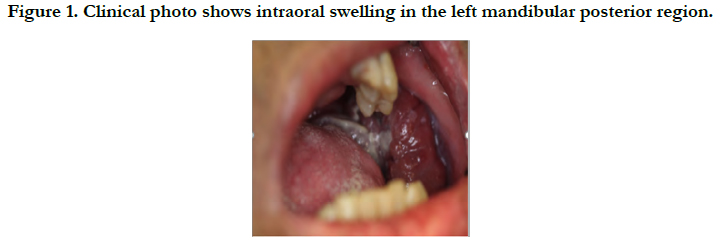
A 57-year-old male patient with a medical history of HIV and pulmonary embolism presented with a three-month history of left facial swelling to the Emergency Room. The patient was given vancomycin and clindamycin before transferring to the emergency department, however, his left facial swelling continued to progress. Clinical examination revealed intraoral swelling in the left mandibular posterior region associated with left inferior alveolar nerve paresthesia (Figure 1).
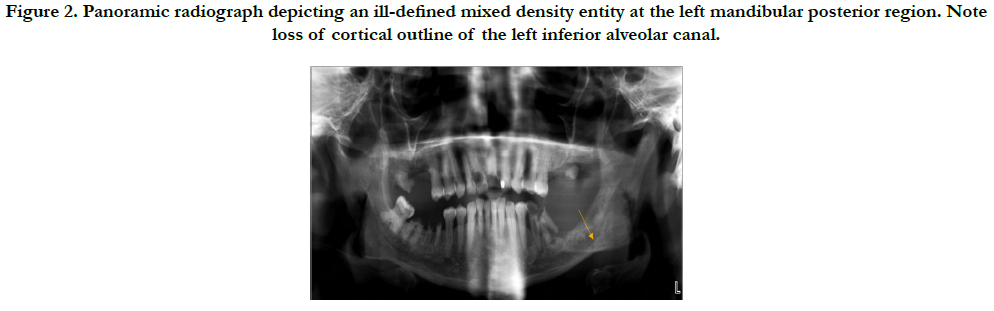
A panoramic study (Orthopantomograph® OP100 D digital panoramic X-ray unit, Instrumentarium Dental, Tuusula, Finland), at 70 kVp, 12 mA, for 17.6 seconds shows an ill-defined, mixed-density area with loss of cortical outline of the inferior alveolar canal in the left mandibular posterior region. Based on the radiographic features and patient history, differential diagnoses of malignancy and inflammatory lesion were made. The study was interpreted by a board-certified oral and maxillofacial radiologist (Figure 2).
Panoramic radiograph depicting a mixed density, ill-defined entity at the left mandibular posterior region. Note loss of cortical outline of the left inferior alveolar canal.
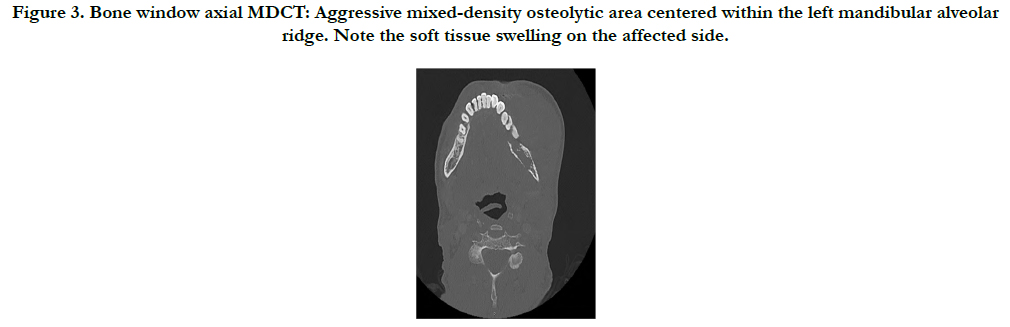
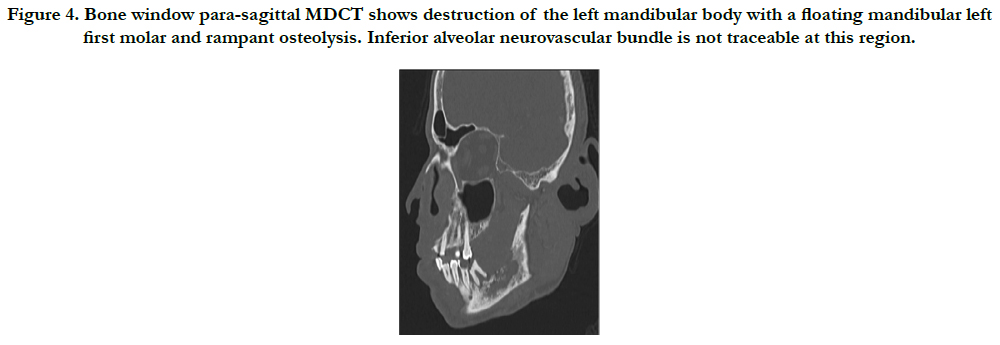
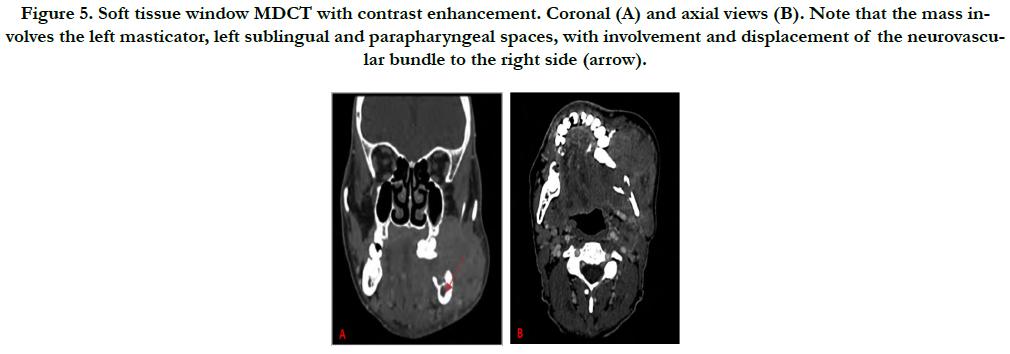
Furthermore, the patient underwent an MDCT examination (Aquilion One, Toshiba America Medical Systems, Tustin, California, USA) that also revealed an ill-defined, mixed-density lytic area in the left posterior mandible with an aggressive soft-tissue mass centered within the alveolar ridge, and involving the masticator, sublingual and left parapharyngeal spaces. A floating tooth is noted as well. The inferior alveolar nerve is involved and displaced to the right. A board-certified neuroradiologist evaluated the dataset and maintained the differential diagnosis (Figure 3-5).
Figure 3. Bone window axial MDCT: Aggressive mixed-density osteolytic area centered within the left mandibular alveolar ridge. Note the soft tissue swelling on the affected side.
Figure 4. Bone window para-sagittal MDCT shows destruction of the left mandibular body with a floating mandibular left first molar and rampant osteolysis. Inferior alveolar neurovascular bundle is not traceable at this region.
Figure 5. Soft tissue window MDCT with contrast enhancement. Coronal (A) and axial views (B). Note that the mass involves the left masticator, left sublingual and parapharyngeal spaces, with involvement and displacement of the neurovascular bundle to the right side (arrow).
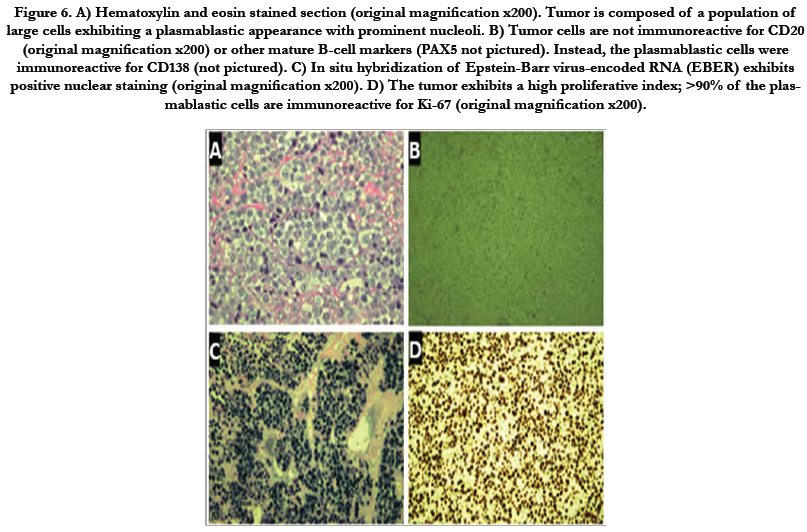
Histopathologic evaluation was performed with histochemical processing and interpretation. Biopsy revealed malignant plasmablastic cells with markedly increased cellular marker (Ki-67) proliferation index (90%), immuno-reactive for transmembrane heparan sulfate proteoglycan (CD138), and diffusely reactive for Epstein-Barr Encoded RNA (EBER). Moreover, malignant plasmablastic cells lacked significant immuonoreactivity for B-cell markers (CD20, CD79a), paired Box 5 (PAX5), common acute lymphocytic leukemia antigen (CALLA)(CD10), B-cell lymphoma 6 (BCL6), neural cell adhesion molecule (NCAM)(CD56), stem cell growth factor (CD117), stem cell marker (CD34), S100, Lysozyme, and T-cell marker (CD3). Overall, these findings were consistent with AIDS-associated PBL (Figure 6).
Figure 6. A) Hematoxylin and eosin stained section (original magnification x200). Tumor is composed of a population of large cells exhibiting a plasmablastic appearance with prominent nucleoli. B) Tumor cells are not immunoreactive for CD20 (original magnification x200) or other mature B-cell markers (PAX5 not pictured). Instead, the plasmablastic cells were immunoreactive for CD138 (not pictured). C) In situ hybridization of Epstein-Barr virus-encoded RNA (EBER) exhibits positive nuclear staining (original magnification x200). D) The tumor exhibits a high proliferative index; >90% of the plasmablastic cells are immunoreactive for Ki-67 (original magnification x200).
The patient received a chemotherapy regimen consisting of dose-adjusted etoposide, prednisone, oncovin (vincristine), cyclophosphamide and hydroxydaunorubicin (doxorubicin) (DA-EPOCH). He completed four cycles of DA-EPOCH; however, the left jaw mass increased in size. Therefore, chemotherapy regimen was adjusted to ifosfamide, carboplatin, etoposide (ICE). After he completed four cycles of ICE, he was scheduled for radiation therapy. He missed his follow-up appointments.
Discussion
PBL has been well known for its frequency in the oral cavity of HIV-positive individuals [10]. However, HIV-positive patients may experience PBL as an extraoral involvement, such as skull, paranasal sinuses, bone, skin, subcutaneous tissues, gastrointestinal tract (GIT), central nervous system, lungs, mediastinum, and various lymph nodes [5]. Lymph nodes, skin and GIT are the most affected extraoral sites [5, 10]. Similarly, oral cavity and GIT are the most involved sites in HIV-negative PBL patients [5]. The estimatee frequency of oral involvement in HIV-positive is 58% whereas in HIV-negative patients is 16% [5]. Oral PBL shows a male predilection with 54.1 average age [9]. Plasmablastic lymphoma has been reported in the elderly patients who are not immuno-compromised [11, 12].
Clinically, oral cavity compromise is the most notable characteristic of PBL in AIDS patients, as noted in our patient who had a large facial mass.
Radiographically, PBL is characterized by an expansile, highly destructive, lytic lesion in the affected areas with overlying swelling of the soft tissues [10]. Moreover; MDCT shows perineural extension, lymphadenopathy as well as infiltration to the adjacent facial spaces as shown in the present case.
Histopathologically, PBL is formed by sheets of large atypical lymphoid cells [10]. The background infiltrate contains small mature lymphocytes mixed with macrophages, which gives an appearance of a ‘starry-sky’ pattern [9]. Immuno-histochemically, the PBL tumor cells are characterized by expression of the plasma cell markers VS38c, CD38, CD138, and multiple myeloma oncogene- 1 (MUM-1) [8]. However, there is an absence of pan B-cell markers CD20 and PAX5 [5, 8]. There is also lacking of T cell marker CD3.8 There is weak expression of leukocyte common antigen (LCA) or CD45, and CD79a [5, 8]. Moreover, the Ki67 index reveals a high proliferation rate [8]. The findings of the present case were consistent with plasmablastic lymphoma.
Clinical and histopathological features of PBL may mimic other malignancies, such as plasmablastic plasmacytoma, immunoblastic diffuse large B-cell lymphoma (immunoblastic-DLBCL), PEL and ALK-DLBCL [5].
Plasmablastic plasmacytoma is inactive neoplasm and most likely localized [13]. It lacks a starry sky pattern and has low rate of Ki67 index [13]. Patients with this neoplasm have responded well to radiation therapy and/ or chemotherapy [13]. Positive CD20 and CD45 immunoreactivity exclude immunoblastic DLBCL from the differential diagnosis [5]. Primary effusion lymphoma (PEL) represents around 4% of all HIV-related non-Hodgkin's lymphomas [14]. PEL is characterized by positive HHV8 while it involves plasmablastic lymphoma if only associated with Castleman disease [14]. Plasmablastic lymphoma most likely occurs in the oral cavity of the HIV patients [10]. It is positive-EBER and negative-ALK [15]. In contrast, ALK-DLBCL has positive ALK but does not seem to be associated with EBV and tends to occur in younger immuno-competent patients [2, 6].
PBL has a poor prognosis with or without treatment [16]. Death may occur in 1-24 months with a 6 months average survival time [16]. The treatment of PBL is mainly through chemotherapy and radiotherapy, with or without surgical excision [5]. PBL’s prognosis is improved by adding highly active antiretroviral therapy (HA-ART) to the treatment [16].
Conclusion
The present case reports radiographic findings of a case of PBL in an immunocompromised patient. Although the definitive diagnosis of PBL relies on biopsy with accurate pathologic and immunohistological testing, awareness of possible diverse findings on MDCT can prevent misdiagnosis and delayed treatment. An oral and maxillofacial radiologist must be aware of this lesion, as it is associated with immune-suppression and could be misconstrued for other pathoses [10]. Immediate referral to oncology is critical for appropriate intervention.
Acknowledgement
The authors would like to thank Dr. Cha, Oral Medicine; Drs. Christopher M Carter, and Wesley M. Hiser, Pathology, College of Medicine, and Dr. Mark Mintline, Oral and Maxillofacial Pathology, for their assistance with histopathologic evaluation and subsequent care to the patient.
References
- England CG, Rui L, Cai W. “Lymphoma: current status of clinical and preclinical imaging with radiolabeled antibodies.” Eur J Nucl Med Mol Imaging. 2017 Mar;44(3):517-532. doi: 10.1007/s00259-016-3560-9. Epub 2016 Nov 14. PubMed PMID: 27844106.
- Campo E, Swerdlow SH, Harris NL, Pileri S, Stein H, Jaffe ES. “The 2008 WHO Classification of Lymphoid Neoplasms and Beyond: Evolving Concepts and Practical Applications.” Blood. 2011 May 12;117(19):5019-32. doi: 10.1182/blood-2011-01-293050. Epub 2011 Feb 7. PubMed PMID: 21300984.
- Cox DP, Treseler P, Dong R, Jordan RC. “Rare oral cavity presentation of a B-cell lymphoblastic lymphoma. A case report and review of the literature.” Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007 Jun;103(6):814-9. Epub 2006 Sep. PubMed PMID: 17531941.
- Delecluse H.J, Anagnostopoulos I, Dallenbach F, Hummel M, Marafioti T, Schneider U, et al. “Plasmablastic Lymphomas of the Oral Cavity: A New Entity Associated With the Human Immunodeficiency Virus Infection.” Blood. 1997 Feb 15;89(4):1413-20. PubMed PMID: 9028965.
- Castillo JJ, Reagan JL. “Plasmablastic lymphoma: a systematic review.” The Scientific World Journal. 2011;11:687-96.
- Beltran B, Castillo J, Salas R, Quiñones P, Morales D, Hurtado F, Riva L, Winer E. “ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature.” Journal of hematology & Oncology. 2009 Dec;2(1):11.
- Hansra D, Montague N, Stefanovic A, Akunyili I, Harzand A, Natkunam Y, et al. “Oral and Extraoral Plasmablastic Lymphoma: Similarities and Differences in Clinicopathologic Characteristics. Am J Clin Pathol. 2010 Nov;134(5):710-9. doi: 10.1309/AJCPJH6KEUSECQLU. PubMed PMID: 20959653.
- Corti M, Minué G, Campitelli A, Narbaitz M, Gilardi L. “An Aggressive Plasmablastic Lymphoma of the Oral Cavity as Primary Manifestation of Acquired Immunodeficiency Syndrome: Case Report and Literature Review.” Int Arch Otorhinolaryngol. 2015 Oct;19(4):354-8. doi: 10.1055/s-0034-1397335. Epub 2015 Jan 8. PubMed PMID: 26491484.
- Sarode SC, Sarode GS, Patil A. “Plasmablastic Lymphoma of the Oral Cavity: A Review.” Oral Oncol. 2010 Mar;46(3):146-53. doi: 10.1016/j.oraloncology. 2009.12.009. Epub 2010 Feb 6. PubMed PMID: 20138565.
- Riedel DJ, Gonzalez-Cuyar LF, Zhao XF, Redfield RR, Gilliam BL. “Plasmablastic Lymphoma of the Oral Cavity: A Rapidly Progressive Lymphoma Associated with HIV Infection.” Lancet Infect Dis. 2008 Apr;8(4):261-7. doi: 10.1016/S1473-3099(08)70067-7. PubMed PMID: 18353267.
- Yamada T, Kitamura N, Sasabe E, Yamamoto T. “Plasmablastic lymphoma of the upper gingiva in an HIV-negative elderly patient.” Oral and Maxillofacial Surgery Cases. 2015 Jun 1;1(2):19-24.
- Hirosawa M, Morimoto H, Shibuya R, Shimajiri S, Tsukada J. “A striking response of plasmablastic lymphoma of the oral cavity to bortezomib: a case report.” Biomarker research. 2015 Dec;3(1):28.
- Loghavi S, Khoury JD, Medeiros LJ. “Epstein–Barr Virus-positive Plasmacytoma in Immunocompetent Patients.” Histopathology. 2015 Aug;67(2):225- 34. doi: 10.1111/his.12640. Epub 2015 Feb 19. PubMed PMID: 25556356.
- Chen Y, Rahemtullahb A, Hochberg E. “Primary Effusion Lymphoma.” Oncologist. 2007 May;12(5):569-76. PubMed PMID: 17522245.
- Nemenqani D, Junainah E, Al-Amoudi S, Junainah J, Mamdouh A, Bajunaid H, Saber A. “ALK positive diffuse large B-cell lymphoma, lymphoplasmablastic differentiation.” Pathology Discov. 2013 Dec 20;1(1):8.
- Bagul N, Mamatha GS, Mahalle A. “Plasmablastic Lymphoma of Gingiva Mimicking a Reactive Lesion: A Case Report.” Case Rep Dent. 2012;2012:259307. Epub 2012 Sep 13. PubMed PMID: 23008784.