Histological and CD31 Immunolocalization after Pulp Therapy using Mta or Portland Cement
Lourenco-Neto N1, Marques NCT2, Prado MTO1, Vitor LLR1, Rodini CO3, Machado MAAM1, Oliveira TM1*
1 Bauru School of Dentistry, Department of Pediatric Dentistry, Orthodontics and Collective Health, University of São Paulo, Bauru, Brazil.
2 UNIFENAS, Department of Pediatric Dentistry, University José RosárioVellano, Alfenas, Brazil.
3 Bauru School of Dentistry, Department of Biology Science, University of São Paulo, Bauru, Brazil.
*Corresponding Author
Thais Marchini Oliveira, DDS, MsC, PhD,
Associate Professor, Bauru School of Dentistry,
University of São Paulo, Alameda Dr. Octávio Pinheiro Brisolla,
9-75, Bauru, São Paulo, 17012-901, Brazil.
Tel: 55 14 32358224
Fax: +55 14 32358082
E-mail: marchini@usp.br
Received: February 20, 2018; Accepted: March 23, 2018; Published: March 24, 2018
Citation:Lourenco-Neto N, Marques NCT, Prado MTO, Vitor LLR, Rodini CO, Oliveira TM, et al., Histological and CD31 Immunolocalization after Pulp Therapy using Mta or Portland Cement. Int J Dentistry Oral Sci. 2018;5(3):622-625. doi: dx.doi.org/10.19070/2377-8075-18000121
Copyright: Oliveira TM©2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
In cases of deep caries or trauma of primary teeth, pulpotomy is the procedure of choice to preserve pulp vitality and the teeth. This study aimed to evaluate the histological analysis and CD31 immunolocalization after pulpotomy using Mineral Trioxide Aggregate or Portland cement in human primary teeth. Twenty mandibular primary molars were divided into two study groups: G1 - Mineral Trioxide Aggregate and G2 - Portland cement. After conventional pulpotomy, clinical and radiographic follow-up, teeth at the regular exfoliation period were extracted for histological analysis and CD31 immunolocalization. The statistical analyses were performed by Kruskal-Wallis followed by Dunn test (P < 0.05). No statistically difference regarding inflammation and blood vessels amount (P > 0.05) were found between groups. The immunohistochemistry analysis revealed positive CD31 expression in the blood vessels of both studied groups. Group 1 showed mainly small blood vessels, while Group 2 exhibited larger blood vessels spread throughout the remaining pulp tissue not related to inflammatory infiltrate. Both materials behaved histologically similar regarding pulp inflammation and vascularization with intense CD31 positivity on the wall of blood vessels.
2.Introduction
3.Material and Methods
3.1 Histological Preparation
3.2 Immunohistochemical Preparation
4.Results
5.Discussion
6.Conclusion
7.Acknowledgments
8.References
Keywords
Dental Pulp; Immunohistochemistry; PECAM-1; Tooth Deciduous.
Introduction
Primary teeth maintenance is critically important for the integrity and aesthetics of the dental arches prior to the eruption of the permanent successors. In cases of deep caries or trauma, pulpotomy is the procedure of choice to preserve pulp vitality and the teeth [1-3]. Nowadays, the dental market has different products indicated for pulp dressing after pulpotomy, although the bio-inductive and regenerative materials are the most indicated, including the mineral trioxide aggregate (MTA) and Portland cement (PC) [2, 4, 5].
Following pulpotomy, MTA exhibited highly successful outcomes, potential for pulp repair, and biocompatibility [2, 5, 6]. Several studies demonstrated that the main components of MTA are similar to those of Portland cement, and the only difference between them is the bismuth oxide present in the MTA formula [4, 7]. Accordingly, PC would be a suitable substitute for MTA [7].
The regenerative functions of the pulp are direct linked to angiogenesis, which helps in the cell migration into the tissue [8-10]. The Cluster of Differentiation 31 (CD31) is a member of the immunoglobulin gene superfamily that mediates cell-cell adhesion through homophilic and heterophilic interactions and transduces intracellular signals that upregulate the function of integrins on leukocytes, activation of platelets and T cells, and angiogenesis [11-13].
The aim of this study was to evaluate the histological analysis and CD31 immunolocalization after pulpotomy using Mineral Trioxide Aggregate or Portland cement in human primary teeth.
Material and Methods
The study protocol was approved by the Institutional Review Board regarding ethical aspects (protocol #121/2009). The clinical procedure, associated risks and benefits were fully explained to the parents or legal guardians of the children. Written informed consent was obtained from the parents or legal guardians of the participants prior to investigation. All the children were screened by taking a detailed history and performing a thorough clinical and radiographic examination.
A total of 20 primary molar teeth in 17 patients were assessed for the study following the inclusion criteria: primary mandibular first or second molars compromised by deep caries with the possibility of proper restoration, vital pulp with no fistula or abscess, absence of internal or external root resorption at radiographic examination, and intraoperatively, only when hemostasis was adequately achieved within 5 minutes after coronal pulp amputation. Exclusion criteria were related to the presence of systemic pathology and history of allergic reaction to local anesthetics or some of the constituents of the dressing materials.
All selected teeth were divided into the two study groups depending on the capping material used on the pulpotomy: MTA or PC. After local anesthesia and rubber dam isolation, caries removal was accomplished with hand piece with a round bur and the opening of the pulp chambers was conducted with high speed and round carbide bur. Coronal pulp tissue was amputated manually with an excavator, followed by irrigation with saline solution until bleeding ceased. These procedures were performed by two previously calibrated pediatric dentists, at one single session as described previously [1].
The amputated pulp stumps and chamber floor were covered with MTA (Angelus, Londrina, PR, Brazil) or PC (Votorantim-Cimentos, São Paulo, SP, Brazil), according to the respective groups. All teeth were restored with reinforced zinc oxide-eugenol (IRM, Dentsply, Petropolis, RJ, Brazil) followed by resin-modified glass ionomer cement (Vitremer, 3M ESPE, São Paulo, SP, Brazil). Then, the patients were dismissed and recalled at periodic followups at three-month intervals, until the treated teeth achieved the regular exfoliation period to be extracted for histological and immunohistochemistry analysis [1, 14].
Following postoperative intervals, the teeth were extracted and immediately fixed in 10% neutral formalin solution for 24 hours, and decalcified in 4% EDTA solution for 45-60 days. Subsequently, each tooth was embedded in paraffin wax and 5 μm-thick serial sections were obtained and stained with hematoxylin and eosin. The histological evaluation was made under a light microscope (Carl Zeiss, Oberkachen, Germany) and based on the scores previously described for inflammation [14]: 0 (none); 1 (mild); 2 (moderate); 3 (intense); and 4 (necrosis); and vascularization: 0 (absent); 1 (regular amount of blood vessels); and 2 (great amount of blood vessels). The data obtained were subjected to statistical analysis by Kruskall-Wallis followed by Dunn test to determine statistically significant differences (P < 0.05).
Tissue sections were digested with Proteinase K (Dako North America, Carpinteria, CA, USA) for 25 minutes, and the endogenous peroxidase activity was blocked with 3% hydrogen peroxide solution in methanol (0.01M) (Easy Path (EP) 12-205222, São Paulo, SP, Brazil) for 10 minutes. Sections were incubated with polyclonal goat anti-human CD31 antibody (Santa Cruz – (M-20) sc - 1506, Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 1:100 dilution for 30 minutes, and then sections were rinsed with phosphate-buffered saline and incubated with a horseradish peroxidase (HRP)- conjugated secondary antibody (Advance, DAKO North America Inc, Carpinteria, CA, USA) for 30 minutes. Further, 3,3’-diaminobenzidine tetra hydrochloride (DAB) solution was used to stain the sections and Lillie-Mayer’s hematoxylin was used to counterstain the sections for 60 seconds.
The statistical analyses were performed by Kruskal-Wallis followed by Dunn test (P<0.05). The CD31 expression in the dentin- pulp complex was descriptively analyzed, emphasizing the presence and formation of blood vessels and the presence or absence of cells related to inflammation and repair of the pulp tissue, under light microscope (Carl Zeiss, Oberkachen, Germany) at x100 magnification.
Results
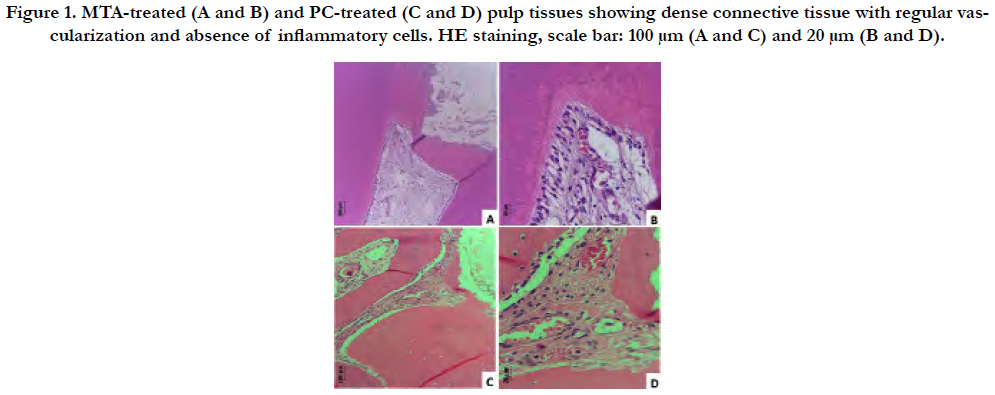
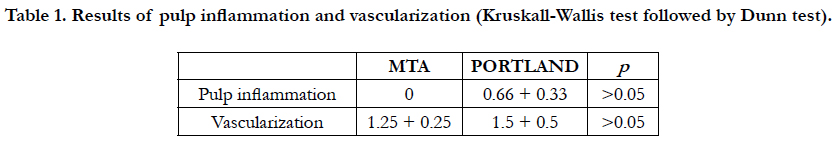
Microscopic descriptive evaluation of MTA-treated and PC-treated pulp tissue showed absence of inflammatory infiltrate within dense connective tissue presenting regular vascularization (Figure 1A-D). No statistically difference regarding inflammation and blood vessels amount (P > 0.05) were found between groups (Table 1).
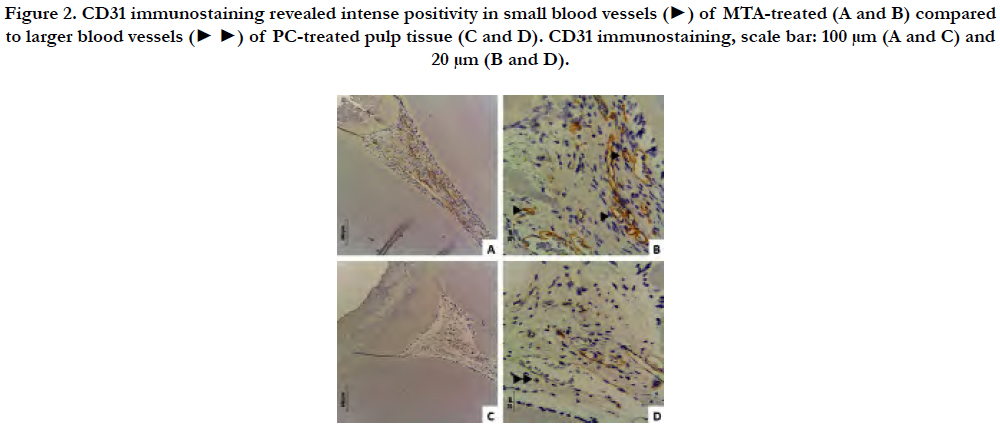
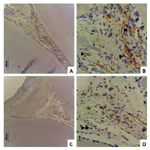
The immunohistochemistry analysis revealed intense positivity for CD31 on the wall of blood vessels from both studied groups not related to inflammatory infiltrate (Figure 2A-D). Specifically, MTA-treated pulp tissue showed mainly small blood vessels (Figure 2A and B) compared to PC-treated pulp tissue, where larger blood vessels were observed spread throughout the remaining pulp tissue (Figure 2C and D).
Figure 1. MTA-treated (A and B) and PC-treated (C and D) pulp tissues showing dense connective tissue with regular vascularization and absence of inflammatory cells. HE staining, scale bar: 100 μm (A and C) and 20 μm (B and D).
Table 1. Results of pulp inflammation and vascularization (Kruskall-Wallis test followed by Dunn test).
Figure 2. CD31 immunostaining revealed intense positivity in small blood vessels (►) of MTA-treated (A and B) compared to larger blood vessels (► ►) of PC-treated pulp tissue (C and D). CD31 immunostaining, scale bar: 100 μm (A and C) and 20 μm (B and D).
Discussion
MTA and PC consist of tricalcium silicate, tricalcium aluminate, calcium silicate, and tetracalcium aluminoferrite that mixed with water form calcium hydroxide [6, 7]. Calcium hydroxide is highly alkaline and when placed directly on the pulp tissue causes superficial necrosis that provokes mild inflammation and a cascade of events aiming at pulp repair [15]. The cascade of pulp repair events begins after pulp amputation and in response to injury to endothelial cells, progenitor pulp cells can be activated by the signaling molecules released by the endothelial cells and migrate to the injury site to initiate inflammatory reactions and the healing process [8-10]. In one hand, angiogenesis is essential for the pulp wound-healing process, because blood vessels account for nutrition and oxygen supply, transport of metabolic waste, pulp homeostasis and metabolism, and stem/progenitor cell migration [15]. On the other hand, pulp fibroblasts play an important role in angiogenesis, because they express angiogenic signals that can act on endothelial cells, namely VEGF and FGF-2 [8].
In this context, the cellular adhesion molecules (CAMs) aid in the interaction of leukocytes with the endothelial cells to initiate the inflammatory response in dental pulp [16]. PECAM-1/CD31, one of these CAMs, plays a role in transmitting mechanicals signals in response to fluid forces in the endothelial cells [11], has a significant impact on endothelial cell-cell and cell-matrix interactions by augmenting cell migration and capillary morphogenesis [12], and is found on the endothelium of all vessels types, justifying its use as immunohistochemical marker of blood vessels, particularly in angiogenesis [12, 17].
The successful treatment with these biocompatible pulp capping materials relies on properties as biocompatibility and low cytotoxicity [7, 18]. The histological analysis of pulpotomized teeth is of extreme importance because the pulp repair process after injury is based on interaction of fibroblast-like cells, inflammatory and immune cells, vascular and perivascular cells, and latent or dormant pulpal stem cells (progenitors) [15, 19].
In this study, the main difference between the materials was the size of the blood vessels showing CD31 immunolocalization. MTA-treated pulp tissue showed small blood vessels, while PCtreated pulp tissue exhibited large blood vessels immunostained by CD31. Although larger blood vessels with intense CD31 reactivity in the subodontoblastic layer and pulp tissue core are linked to inflamed pulp [15, 20], both groups exhibited normal histological signs as absence of inflammatory infiltrate within dense connective tissue showing regular vascularization. Large blood vessels and intense expression of adhesion molecules in vascular tissue favor the increase of tissue fluid with inflammatory cells, especially leukocytes, a critical step in the beginning of the immunological response to tissue inflammation [16, 20]. These results reaffirm the biocompatibility of both materials showed by longterm clinical and radiographic success [1-3].
Conclusion
This study found the suitability of both MTA and PC as pulp capping materials in the pulpotomy of primary teeth, because both materials behaved histologically similar regarding pulp inflammation and vascularization with intense positivity for CD31 on the wall of blood vessels.
Acknowledgments
The authors would like to thank all the volunteers for their participation in this study, and the excellent laboratorial assistance of Daniele Santi Ceolin and Patrícia De Sá Mortágua Gemino. This work was supported by Sao Paulo Research Foundation (FAPESP) [grant numbers 2009/11284-4] to TMO.
References
- Lourenço Neto N, Marques NC, Fernandes AP, et al. Immunolocalization of dentin matrix protein‐1 in human primary teeth treated with different pulp capping materials. J Biomed Mater Res B Appl Biomater. 2016 Jan;104(1):165-9. PubMed PMID: 25678029.
- Oliveira TM, Moretti AB, Sakai VT, Neto NL, et al. Clinical, radiographic and histologic analysis of the effects of pulp capping materials used in pulpotomies of human primary teeth. Eur Arch Paediatr Dent. 2013 Apr;14(2):65-71. PubMed PMID: 23549993.
- Sakai VT, Moretti AB, Oliveira TM, Fornetti AP, Santos CF, Machado MA, et al., Pulpotomy of human primary molars with MTA and Portland cement: a randomised controlled trial. Br Dent J. 2009 Aug 8;207(3):E5. PubMed PMID: 19629145.
- Bhagat D, Sunder RK, Devendrappa SN, Vanka A, Choudaha N. A comparative evaluation of ProRoot mineral trioxide aggregate and Portland cement as a pulpotomy medicament. J Indian Soc Pedod Prev Dent. 2016 Apr-Jun;34(2):172-6. PubMed PMID: 27080969.
- Moretti AB, Sakai VT, Oliveira TM, Fornetti AP, et al. The effectiveness of mineral trioxide aggregate, calcium hydroxide and formocresol for pulpotomies in primary teeth. Int Endod J. 2008 Jul;41(7):547-55. PubMed PMID: 18479381.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part I: chemical, physical, and antibacterial properties. J Endod. 2010 Jan;36(1):16-27. PubMed PMID: 20003930.
- Steffen R, van Waes H. Understanding mineral trioxide aggregate/Portlandcement: a review of literature and background factors. Eur Arch Paediatr Dent. 2009 Jun;10(2):93-7. PubMed PMID: 19627674.
- Tran-Hung L, Mathieu S, About I. Role of human pulp fibroblasts in angiogenesis. J Dent Res. 2006 Sep;85(9):819-23. PubMed PMID: 16931864.
- Tecles O, Laurent P, Zygouritsas S, et al. Activation of human dental pulp progenitor/stem cells in response to odontoblast injury. Arch Oral Biol. 2005 Feb;50(2):103-8. PubMed PMID: 15721135.
- Mathieu S, El-Battari A, Dejou J, et al. Role of injured endothelial cells in the recruitment of human pulp cells. Arch Oral Biol. 2005 Feb;50(2):109-13. PubMed PMID: 15721136.
- Privratsky JR, Newman DK, Newman PJ. PECAM-1: conflicts of interest in inflammation. Life Sci. 2010 Jul 17;87(3-4):69-82. PubMed PMID: 20541560.
- Privratsky JR, Newman PJ. PECAM-1: regulator of endothelial junctional integrity. Cell Tissue Res. 2014 Mar;355(3):607-19. PubMed PMID: 24435645.
- Delisser HM, Baldwin HS, Albelda SM. Platelet Endothelial Cell Adhesion Molecule 1 (PECAM-1/CD31): A Multifunctional Vascular Cell Adhesion Molecule. Trends Cardiovasc Med. 1997 Aug;7(6):203-10. PubMed PMID: 21235886.
- Marques NC, Neto NL, Rodini Cde O, et al. Low-level laser therapy as an alternative for pulpotomy in human primary teeth. Lasers Med Sci. 2015 Sep;30(7):1815-22. PubMed PMID: 25240388.
- Goldberg M, Njeh A, Uzunoglu E. Is Pulp Inflammation a Prerequisite for Pulp Healing and Regeneration? Mediators Inflamm. 2015;2015:347649. PubMed PMID: 26538825.
- Bagis B, Atilla P, Cakar N, Hasanreisoglu U. An immunohistochemical evaluation of cell adhesion molecules in human dental pulp after tooth preparation and application of temporary luting cements. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 Jan;107(1):137-44. PubMed PMID: 19101496.
- DeLisser HM, Newman PJ, Albelda SM. Molecular and functional aspects of PECAM-1/CD31. Immunol Today. 1994 Oct;15(10):490-5. PubMed PMID: 7945775.
- Parirokh M, Torabinejad M. Mineral trioxide aggregate: a comprehensive literature review-part III: clinical applications, drawbacks, and mechanism of action. J Endod. 2010 Mar;36(3):400-13. PubMed PMID: 20171353.
- Goldberg M, Farges JC, Lacerda-Pinheiro S, Six N, Jegat N, Decup F, Septier D, Carrouel F, Durand S, Chaussain-Miller C, DenBesten P. Inflammatory and immunological aspects of dental pulp repair. Pharmacol Res. 2008 Aug;58(2):137-47. PubMed PMID: 18602009.
- Sawa Y, Yoshida S, Shibata KI, Suzuki M, Mukaida A. Vascular endothelium of human dental pulp expresses diverse adhesion molecules for leukocyte emigration. Tissue Cell. 1998 Apr;30(2):281-91. PubMed PMID: 9661300.