Effect of Oral Infections on Serum Creatinine Levels in Diabetic Rats
Cintra LT1*, Facundo AC1, Valentim D1, Prieto AK1, Silva CO2, Sumida DH3, Bomfim SR4, Dezan-Júnior E1, Gomes-Filho JE1
1* Endodontics, Araçatuba Dental School, Univ Estadual Paulista - UNESP, SP, Brazil
2* Periodontics, Maringá Dental School, Univ Estadual Maringá - UEM, PR, Brazil
3* Basic Science, Araçatuba Dental School, Univ Estadual Paulista - UNESP, SP, Brazil.
4* Clinic and Surgery and Animal Reproduction, Araçatuba Veterinary Medicine School, Univ Estadual Paulista -UNESP, SP, Brazil
*Corresponding Author
Luciano Tavares Angelo Cintra
Endodontics, Araçatuba Dental School,
Univ Estadual Paulista - UNESP, SP, Brazil.
Tel: (0055) 18 36363252
Fax: (0055) 18 36363279
E-mail: lucianocintra@foa.unesp.br
Article Type: Research Article
Received: Aprial 16, 2013; Accepted: May 16, 2013; Published: May 20, 2013
Citation: Cintra LT et al., (2013) Effect of Oral Infections on Serum Creatinine Levels in Diabetic Rats. Int J Diabetol Vasc Dis Res. 1(3), 19- 23. doi: dx.doi.org/10.19070/2328-353X-1300026.
Copyright: Cintra LT© 2014. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: The aim of this study was to evaluate the relationship between pulpal and/or periodontal disease and serum creatinine levels in a rat model of diabetes mellitus.
Methods: Eighty male rats (Rattus norvegicus albinus, Wistar) were divided into the following 8 groups comprising 10 animals each: normal (G1), with pulpal disease (G2), with periodontal disease (G3), with both pulpal and periodontal disease (G4), diabetic (G5), diabetic with pulpal disease (G6), diabetic with periodontal disease (G7),and diabetic with both pulpal and periodontal disease (G8). Diabetes was induced by injecting streptozotocin, pulpal disease were induced by exposing pulpal tissue to the oral environment, and periodontal disease was induced by periodontal ligature. After 30 days, blood was collected by cardiac puncture and the animals were killed. The maxillae were processed for histopathology. Serum creatinine levels were measured by the enzymatic method. The total assessed values were statistically analyzed by analysis of variance and Tukey’s test (p < 0.05).
Results: Serum creatinine levels were significantly higher in diabetic rats than that in all normoglycemic rats (p<0.05). The presence of pulpal and periodontal disease increased the serum creatinine levels in normoglycemic and diabetic rats, but there was no statistical difference between the groups (p > 0.05).
Conclusions: We found that the serum creatinine level was higher in diabetic rats and may be related to the presence of oral infections.
Clinical significance: Changes in serum creatinine level may be related to the presence of oral infections and diabetes.
2.Introduction
3.Methods
2.1 Induction of experimental diabetes
2.2 Induction of oral infections
2.3 Assessment of serum creatinine levels
2.4 Histopathological analysis
3.Results
4.Discussion
5.Acknowledgment
6.References
Key Words
Diabetes; Apical Periodontitis; Periodontal Disease; Creatinine Level.
Introduction
DOral infections such as pulpal and periodontal disease are frequently associated with diabetes mellitus [1-3]. Periodontal disease is one of the most prevalent complications in diabetic patients [3-5]. It is also evident in the increased severity of chronic periodontitis, periodontal abscess, and predisposition to infections in these individuals [6, 7]. The reverse is also true, as periodontal infection is capable of exacerbating a diabetic condition, which is another important aspect to be considered [8].
The current literature is replete with studies linking diabetes mellitus to periodontal disease alone [7, 9-11]. However, information on pulpal disease, which is characterized by apical periodontitis due to infectious necrosis of the pulp, is still lacking [1, 12, 13].
The effect of diabetes mellitus in apical periodontitis has been investigated in animal models [14, 15]. It was observed that diabetes accelerates the development and progression of periapical periodontitis in rats, as well as the relationship of periodontal disease [16]. However, information on the association between pulpal and periodontal disease with diabetes is limited to a few studies [1, 17], and the effects on systemic conditions have not been studied in detail [1].
One of the systemic complications for diabetic patients is renal dysfunction [18], which can be diagnosed by measuring urea and creatinine levels in the blood [19]. Diabetic nephropathy, a major long-term complication of diabetes mellitus, is the most common cause of end-stage renal disease worldwide requiring dialysis, and it is becoming a challenge to public healthcare systems [20, 21].
Creatinine is the preferred marker for renal dysfunction and is readily available in clinical laboratory settings [22]. Being an indicator of decreased kidney function, serum creatinine levels are largely unaffected by a typical diet, and are thus used as markers of renal function [23, 24]. Creatinine is a byproduct of muscle metabolism and typically remains in a steady state balanced out by renal elimination [22]. Despite their limiCreatinine is the preferred marker for renal dysfunction and is readily available in clinical laboratory settings [22]. Being an indicator of decreased kidney function, serum creatinine levels are largely unaffected by a typical diet, and are thus used as markers of renal function [23, 24]. Creatinine is a byproduct of muscle metabolism and typically remains in a steady state balanced out by renal elimination [22]. Despite their limitations, creatinine levels are currently used in clinical practice to estimate the glomerular filtration rate because it is inexpensive, harmless, and easy to perform in patients [25].
High creatinine levels can relate to other infections such as acute infectious meningitis [25]. It has been suggested that creatinine levels increase due to glomerular hyperperfusion [26]. Experimental studies have shown that high creatinine levels are observed during the initial hyperkinetic phase of sepsis [27, 28].
Lower serum creatinine represents a greater risk for developing diabetes [29]. The relationship between chronic kidney disease, diabetes, and oral health status has been reported [30]. Nephropathy is a chronic complication of diabetes mellitus and may be related to periodontal disease [31, 32]. It is known that periodontal disease causes changes in serum creatinine levels [33]; however, to our knowledge, the association of this condition with diabetes or pulpal disease has not been explored thus far.
Thus, it seemed pertinent to evaluate the relationship between pulpal and/or periodontal disease and serum creatinine levels in a rat model of diabetes mellitus.
Methods
Eighty male Rattus norvegicus albinus Wistar rats, each weighing 250–280 g, were used in the study. The animals were housed in temperature-controlled rooms and given ad libitum access to water and food. The institutional ethical committee approved the experimental protocol, which was conducted in accordance with the institutional ethical committee guidelines. The animals were divided into 8 groups of 10 animals each,as shown in Table 1.
The animals were intramuscularly anesthetized with ketamine (87 mg/kg; Francotar; Virbac do Brasil Ind. e Com. Ltda., Roseira, Brazil) and xylazine (13 mg/kg; Rompun; Bayer S.A., Sao Paulo, Brazil). The rats were randomly assigned into groups and were endovenously injected in the penile vein with either citrate buffer solution (0.01 M, pH 4.5; groups 1–4, n = 40) or with streptozotocin (Sigma-Aldrich Corp., St. Louis, MO, USA). Streptozotocin was dissolved in citrate buffer solution at 65 mg/kg body weight for experimental induction of diabetes (groups 5–8, n = 40) [34].
Six days after diabetes mellitus was induced, blood samples were collected from each animal to determine their blood glucose levels. Rats with blood glucose levels exceeding 250 mg/dL were used in the study [35].
For the development of apical periodontitis, the pulps of the right upper first molars were exposed on the mesial surface using surgical round burs (Broca LN Long Neck; Maillefer, Dentsply Ind. e Com. Ltda, Petrópolis, Brazil) (groups 2, 4, 6, and 8) [36]. To induce periodontal disease in rats from groups 3, 4, 7, and 8, sterile 4/0 silk ligatures (Ethicon; Johnson & Johnson, São Paulo, Brazil) were placed around the left second maxillary molar [1].
To determine serum creatinine levels, venous blood samples (1 mL) were collected via cardiac puncture after 30 days, and the animals were killed with an overdose of the anesthetic solution.
The samples were placed in EDTA and immediately transferred to a technician who was blinded to case status. Serum creatinine was measured by the Jaffe method [19, 24, 25, 29]. The Jaffé reaction is based on the yellow-orange color produced by creatinine reacting with alkaline picrate, which is measured at 492 nm. The amount of color formed is proportional to the concentration of creatinine in the sample. The sensitivity of the Jaffé reaction is 0.5 mg/dL creatinine in samples, and the entire detection time spans approximately 30 min (Creatinina K Labtest®, Labtest Diagnóstica S.A., Brazil).
The total assessed values were tabulated for each experimental group, and a single calibrated operator analyzed the data in a blinded manner. One-way analysis of variance and Tukey’s test were used for statistical analysis, and a significance level of 5% (p < 0.05) was used to compare the mean values.
Maxillae were removed and immediately placed in 10% bbuffered formalin. Specimens were rinsed in sterile water and decalcified in 10% EDTA. They were rinsed again in sterile water, dehydrated in ethanol, cleared in xylene, and embedded in paraffin [1]. Six micron-thick serial sections of the molars and surrounding tissues were prepared and stained with hematoxylin and eosin (HE). We made serial 6-μm thick paraffin sections of the mesial–distal aspects of all upper molars, and these were stained with HE. Morphological studies of the periapical and periodontal area were conducted.
Results
Thirty days after pulp exposure, pulps exhibited total necrosis and periapical lesions were established. Moderate to severe inflammatory reaction composed primarily of neutrophils (polymorphonuclear cells) and mononuclear cells was observed (Fig. 1a, b).
Figure 1. Histological overview (a, b) Intense acute inflammatory response (black arrows), tissue disorganization, and substantial bone resorption is observed adjacent to the tooth apex region (HE, original magnification ×25, inset magnification ×100). (c, d) Histological evidence of intense inflammatory cell concentration, substantial bone loss, and root resorption (*) (HE, original magnification ×25, inset magnification ×100).
N, total necrosis, AB, alveolar bone, C, cementum, D, dentin; arrowheads, inflammatory infiltrate,*, root resorption
Periodontal disease was observed based on the presence of inflammatory cells and by measuring the distance from the cemento-enamel junction to the alveolar bone crest at the palatal surface of the midmesiopalatal root region of the second molar under ×100 magnification. We observed evidence of aggressive periodontitis with substantial periodontal bone loss (Fig. 1c, d).
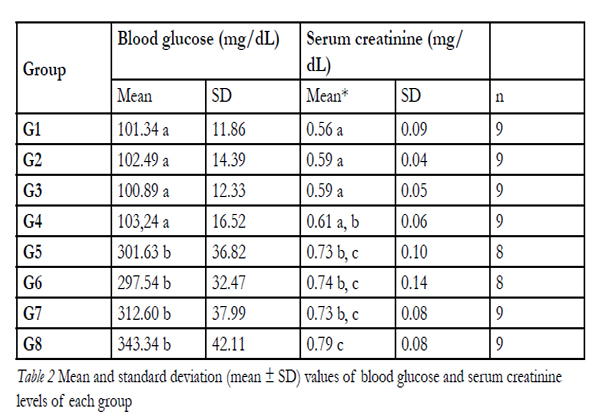
Blood glucose levels were significantly increased in diabetic rats as compared to that in control rats, indicating development of hyperglycemia (Table 2). The changesin the serum creatinine levels of non-diabetic and diabetic rats are shown in Table 2.
Total serum creatinine levels were similar in the nondiabetic(p > 0.05) and in diabetic groups (p > 0.05).Conversely, diabetic rats had significantly higher serum creatinine levels than the control rats (p = 0.000) (Table2).
Table 2. Mean and standard deviation (mean ± SD) values of blood glucose and serum creatinine levels of each group.
Diabetic rats with pulpal and periodontal disease (G8)had high levels of serum creatinine, which was statistically significant compared to all non-diabetic rat groups (G1–G4). Normoglycemic rats with pulpal and periodontal disease (G4) had serum creatinine levels similar to those of diabetic rats without oral infection (G5) (Table 2).
Discussion
There are a number of different rodent models for diabetes, particularly type 1 diabetes. Two models are commonly used: streptozotocin-induced diabetes in rats and non-obese diabetic (NOD) mice [37]. The advantages of the streptozotocin model are that diabetes can be induced in a controlled manner in all animals, and animals in the diabetes and non-diabetes groups are of the same genetic background [38]. In this study,blood glucose levels were higher in rats in the diabetic model group than in those in the normal control group, indicating that hyperglycemia persisted in the diabetic rats.
Two models of oral infection were used [1]. Our results are in agreement with that of other studies [14, 39], which demonstrated histologically that induced periradicular lesions in diabetic rats were larger than that in non-diabetic rats. The induction of experimental periodontitis stimulated inflammatory infiltrate formation in both diabetic and normoglycemic rats. In summary, diabetic rats had a higher degree of inflammation and greater periodontal bone loss through enhanced resorption. These results also agree with that of previous studies [40, 41].
We measured creatinine levels in the rat serum with the Jaffé method, similar to other studies [19, 24, 25, 29]. The traditional method for detecting creatinine is based on a modified Jaffé reaction, which is widely used in both laboratory and clinical detection. The entire detection time requires approximately 30 min. The reaction is carried out for a fixed period in order to minimize the interference of other substances reacting with the picric acid [42].
Creatinine is transported by the bloodstream to the kidney. There is little to no tubular reabsorption of creatinine by the kidney tubules. Therefore, creatinine levels in the blood may be used to calculate creatinine clearance, which reflects the glomerular filtration rate. Any condition that impairs the function of the kidney will likely increase the creatinine level in the blood. Changes in creatinine levels have been linked with diabetes [29, 43, 44], periodontal disease [33], acute infectious meningitis [25], hyperkinetic phase of sepsis [23, 24], kidney disease, kidney transplant, and oral health status [30, 45, 46].
In the present study, serum creatinine levels were significantly elevated in the diabetic group (0.73 ± 0.10 mg/dL) compared with that in the control group (0.56 ± 0.09 mg/dL). These results are also in agreement with that of previous studies [43, 47]. These results confirm that streptozotocin-induced diabetes is a factor for changes in creatinine levels. Conversely, lower serum creatinine levels increased the risk of type 2 diabetes [29].
Some studies have shown that periodontal conditions may affect renal function [33, 48-50]. However, severe periodontitis was not correlated with renal dysfunction in a studied population [51]. This difference between these studies may be related to methodology.
There is no related study regarding the relationship to pulpal infection. In the present study, a higher mean serum creatinine level was associated with pulpal and periodontal disease in normal and diabetic rats (Table 2). In addition, a recent report demonstrated that pulpal and periodontal disease increase triglyceride levels in diabetic rats, confirming the hypothesis that endodontic infections influence systemic health when associated with periodontal disease [1].
We found that the presence of pulpal and periodontal disease increased serum creatinine levels in normoglycemic and diabetic rats; however, no statistical differences were found (p > 0.05). In conclusion, the present results suggest that serum creatinine levels are higher in diabetic rats and may be related to the presence of oral infections.
Acknowledgements
This study was supported by PROPe/UNESP (005/2011).
Conflict of Interest
The authors declare that they have no conflicts of interest to declare.
References
- Cintra LT, Facundo ACS, Azuma MM, Sumida DH, Astolphi RD, Bomfim SR, et al. Pulpal and periodontal diseases increase triglyceride levels in diabetic rats. Clinical Oral Investigation 2012; published online: 05 October 2012.
- Marotta PS, Fontes TV, Armada L, Lima KC, Rôças IN, Siqueira JFJr. Type 2 diabetes mellitus and the prevalence of apical periodontitis and endodontic treatment in an adult Brazilian population. J Endod 2012;38(3):297-300.
- Awuti G, Younusi K, Li L, Upur H, Ren J. Epidemiological survey on the prevalence of periodontitis and diabetes mellitus in uyghur adults from rural hotan area in xinjiang. Exp Diabetes Res 2012;2012:758921.
- Sandberg GE, Sundberg HE, Fjellstrom CA, Wikblad KF. Type 2 diabetes and oral health: a comparison between diabetic and nondiabetic subjects. Diabetes Res Clin Pract 2000;50(1):27-34.
- Valerio MA, Kanjirath PP, Klausner CP, Peters MC. A qualitative examination of patient awareness and understanding of type 2 diabetes and oral health care needs. Diabetes Res Clin Pract 2011;93(2):159-65.
- Jimenez M, Hu FB, Marino M, Li Y, Joshipura KJ. Type 2 diabetes mellitus and 20 year incidence of periodontitis and tooth loss. Diabetes Res Clin Pract 2012 Oct 3. doi:pii: S0168-8227(12)00362-2. 10.1016/j.diabres.2012.09.039. [Epub ahead of print].
- Preshaw PM, Alba AL, Herrera D, Jepsen S, Konstantinidis A, Makrilakis K, et al. Periodontitis and diabetes: a two-way relationship. Diabetologia 2012;55(1):21-31.
- Lakschevitz F, Aboodi G, Tenenbaum H, Glogauer M. Diabetes and periodontal diseases: interplay and links. Curr Diabetes Rev 2011;7(6):433-9.
- Zhang L, Li X, Bi LJ. Alterations of collagen-I, MMP-1 andTIMP-1 in the periodontal ligament of diabetic rats under mechanical stress. J Periodontal Res 2011;46(4):448-55.
- . Chang PC, Chung MC, Wang YP, Chien LY, Lim JC, Liang K,et al. Patterns of Diabetic Periodontal Wound Repair: A Study Utilizing Micro-Computed Tomography and Immunohistochemistry. J Periodontol 2011 Oct 3. [Epub ahead of print]
- Ou L, Li RF. Effect of periodontal treatment on glycosylated hemoglobin levels in elderly patients with periodontal disease and type 2 diabetes. Chin Med J (Engl) 2011;124(19):3070-3.
- Bender IB, Bender AB. Diabetes mellitus and the dental pulp. J Endod 2003; 29(6):383-9.
- Kodama Y, Matsuura M, Sano T, Nakahara Y, Ozaki K, et al. Diabetes enhances dental caries and apical periodontitis in caries-susceptible WBN/KobSlcRats. Comp Med 2011;61(1):53-9.
- Kohsaka T, Kumazawa M, Yamasaki M, Nakamura H. Periapical lesions in rats with streptozotocin-induced diabetes. J Endod 1996;22:418-21.
- Iwama A, Nishigaki N, Nakamura K, Imaizumi I, Shibata N, Yamasaki M, et al. The effect of high sugar intake on the development of periradicular lesions in rats with type 2 diabetes. J Dent Res 2003;82(4):322-5.
- Pepelassi E, Xynogala I, Perrea D, Agrogiannis G, Pantopoulou A, Patsouris E, et al. Histometric assessment of the effect of diabetes mellitus on experimentally induced periodontitis in rats. J Int Acad Periodontol 2012;14(2):35-41.
- Schulze A, Schönauer M, Busse M. Sudden improvement of insulin sensitivity related to an endodontic treatment. J Periodontol 2007;78(12):2380-4.
- Unsal A, Koc Y, Basturk T, Akgun AO, Sakaci T, Ahbap E. Risk factors for progression of renal disease in patient with diabetic nephropathy. Eur Rev Med Pharmacol Sci 2012;16(7):878-83.
- Bansal N, Whooley MA, Regan M, McCulloch CE, Ix JH, Epel E, Blackburn E, Lin J, Hsu CY. Association between kidney function and telomere length: the heart and soul study. Am J Nephrol. 2012;36(5):405- 11.
- King H, Aubert R, Herman, W., Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projection. Diabetes Care 1998;21:1414–1431.
- Hayden MR, Whaley-Connell A, Sowers JR. Renal redox stress and remodeling in metabolic syndrome, type 2 diabetes mellitus, and diabetic nephropathy: paying homage to the podocyte. Am J Nephrol. 2005 Nov-Dec;25(6):553-69.
- Wei F, Cheng S, Korin Y, Reed EF, Gjertson D, Ho CM, et al. Serum creatinine detection by a conducting-polymer-based electrochemical sensor to identify allograft dysfunction. Anal Chem 2012;84(18):7933- 7.
- Marcotte L, Godwin M. Natural history of elevated creatinine levels. Can Fam Physician 2006;52(10):1264-5.
- Sato KK, Hayashi T, Harita N, Koh H, Maeda I, Endo G, Nakamura Y, Kambe H, Fukuda K. Elevated white blood cell count worsens proteinuria but not estimated glomerular filtration rate: the Kansai Healthcare Study. Am J Nephrol. 2011;34(4):324-9.
- Lautrette A, Phan TN, Ouchchane L, Aithssain A, Tixier V, Heng AE, et al. High creatinine clearance in critically ill patients with community-acquired acute infectious meningitis. BMC Nephrol 2012;13(1):124.
- Praga M: Synergy of low nephron number and obesity: a new focus on hyperfiltration nephropathy. Nephrol Dial Transplant 2005;20(12):2594–2597.
- Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow. Intensive Care Med 2002;28(12):1804–1809.
- Wan L, Bellomo R, May CN: The effects of normal and hypertonic saline on regional blood flow and oxygen delivery. Anesth Analg 2007;105(1):141–147.
- Harita N, Hayashi T, Sato KK, Nakamura Y, Yoneda T, Endo G, et al. Lower serum creatinine is a new risk factor of type 2 diabetes: the Kansai healthcare study. Diabetes Care 2009;32(3):424-6.
- Akar H, Akar GC, Carrero JJ, Stenvinkel P, Lindholm B. Systemic consequences of poor oral health in chronic kidney disease patients. Clin J Am Soc Nephrol 2011;6(1):218-26.
- Pontes Andersen CC, Holmstrup P, Buschard K, Flyvbjerg A. Renal alterations in prediabetic rats with periodontitis. J Periodontol 2008;79(4):684-90.
- Grubbs V, Plantinga LC, Crews DC, Bibbins-Domingo K, Saran R, Heung M, et al; centers for disease control and prevention CKD surveillance team. Vulnerable populations and the association between periodontal and chronic kidney disease. Clin J Am SocNephrol 2011;6(4):711-7.
- Yoshihara A, Hayashi Y, Miyazaki H. Relationships among bone turnover, renal function and periodontal disease in elderly Japanese. J Periodontal Res 2011;46(4):491-6.
- Nicholas SB, Mauer M, Basgen JM, Aguiniga E, Chon Y: Effect of angiotensin II on glomerular structure in streptozotocin-induced diabetic rats. Am J Nephrol 2004 Sep-Oct;24(5):549-56.
- Rosenberger C, Khamaisi M, Goldfarb M, Shina A, Shilo V,Zilbertrest F, et al: Acute kidney injury in the diabetic rat: studies in theisolated perfused and intact kidney. Am J Nephrol 2008;28(5):831-9.
- Garber SE, Shabahang S, Escher AP, Torabinejad M. The effect of hyperglycemia on pulpal healing in rats. J Endod 2009;35(1):60-2.
- Kachapati K, Adams D, Bednar K, Ridgway WM. The Non- Obese Diabetic (NOD) Mouse as a Model of Human Type 1 Diabetes. Methods Mol Biol 2012;933:3-16.
- Fouad AF. Diabetes mellitus as a modulating factor of endodontic infections. J Dent Educ 2003;67(4):459-67.
- Armada-Dias L, Breda J, Provenzano JC, Breitenbach M, Rôças IN, Gahyva SM, et al. Development of periradicular lesions in normal and diabetic rats. J Appl Oral Sci 2006; 14(5):371-5.
- Salvi GE, Kandylaki M, Troendle A, Persson GR, Lang NP. Experimental gingivitis in type 1 diabetics: a controlled clinical and microbiological study. J ClinPeriodontol 2005;32(3):310-6.
- Liu R, Bal HS, Desta T, Krothapalli N, Alyassi M, Luan Q, etal. Diabetes enhances periodontal bone loss through enhanced resorption and diminished bone formation. J Dent Res 2006;85(6):510-4.
- Spanaus KS, Kollerits B, Ritz E, Hersberger M, Kronenberg F, von Eckardstein A. Mild and Moderate Kidney Disease (MMKD) Study Group. Serum creatinine, cystatin C, and beta-trace protein in diagnostic staging and predicting progression of primary nondiabetic chronic kidney disease. Clin Chem 2010;56(5):740-9.
- Omara EA, Nada SA, Farrag AR, Sharaf WM, El-Toumy SA. Therapeutic effect of Acacia nilotica pods extract on streptozotocin induced diabetic nephropathy in rat. Phytomedicine 2012;19(12):1059-67.
- Li F, Yang N, Zhang L, Tan H, Huang B, Liang Y, Chen M, Yu X. Increased expression of toll-like receptor 2 in rat diabetic nephropathy. Am J Nephrol. 2010;32(2):179-86.
- Vaishya R, Singh J, Lal H .Effect of irbesartan on streptozotocin- induced diabetic nephropathy: an interventionary study. Indian J ClinBiochem 2008;23:195–197
- Adibelli Z, Woo E, Abt P, Goral S. Elevated serum creatinine in a kidney transplant recipient: unusual suspect. ClinNephrol 2012;78(4):322-4.
- Arya A, Yadav HN, Sharma PL. Involvement of vascular endothelial nitric oxide synthase in development of experimental diabetic nephropathy in rats. Mol Cell Biochem 2011;354(1-2):57-66. Epub 2011Apr 6.
- Kshirsagar AV, Moss KL, Elter JR, Beck JD, Offenbacher S, Falk RJ. Periodontal disease is associated with renal insufficiency in the Atherosclerosis Risk In Communities (ARIC) study. Am J Kidney Dis 2005;45:650–657.
- Davidovich E, Schwarz Z, Davidovitch M, Eidelman E, Bimstein E. Oral findings and periodontal status in children, adolescents and young adults suffering from renal failure. J ClinPeriodontol 2005;32: 1076–1082.
- Yoshihara A, Deguchi T, Hanada N, Miyazaki H. Renal function and periodontal disease in elderly Japanese. J Periodontol (2007);78(7):1241-8.
Brotto RS, Vendramini RC, Brunetti IL, Marcantonio RA, Ramos AP, Pepato MT. Lack of Correlation between Periodontitis and Renal Dysfunction in Systemically Healthy Patients. Eur J Dent 2011;5(1):8-18.