Ziziphus Joazeiro Martius and Ketoconazole added to Acrylic Resin Present Antifungal Activity
Neves-Silva R1*, Machado JL2
1 Ribeirão Preto Dental School, Department of Stomatology, Public Oral Health, and Forensic Dentistry, Oral Pathology Area, University of São Paulo (FORP/USP), Ribeirão Preto, São Paulo, Brazil.
2 School of Dentistry, Oral Medicine Department, Federal University of Alagoas (FO/UFAL), Maceió, Alagoas, Brazil.
*Corresponding Author
Rodrigo Neves-Silva, DDS, MSc, PhD,
Oral Pathology Area, Department of Stomatology, Public Oral Health, and Forensic Dentistry,
Ribeirão Preto Dental School, University of São Paulo (FORP/USP), Avenida do Café,
S/N, Zipcode: 14040-904. Ribeirão Preto, SP, Brazil.
Tel: 55 16 3315 4011
Fax: 55 16 3315 4102
Email: rodrigoneves.rn@hotmail.com
Recieved: December 23, 2017; Accepted: January 11, 2018; Published: January 19, 2018
Citation: Neves-Silva R, Machado JL. Ziziphus joazeiro Martius and Ketoconazole added to Acrylic Resin Present Antifungal Activity. Int J Microbiol Adv Immunol. 2018;06(1):86-91. doi: http://dx.doi.org/10.19070/2329-9967-1800017
Copyright: Neves-Silva R© 2018. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
C. albicans has been shown to be associated with denture-related stomatitis. This study aimed to evaluate the antifungal effect of a hydroalcoholic extract of Ziziphus joazeiro Martius (HEZJM) and ketoconazole. Ninety specimens were used, 10 in each group: GROUP I – 1% of the polymer replaced by HEZJM; GROUP II – 2% of the polymer replaced by HEZJM; GROUP III – 1% HEZJM added to the polymer; GROUP IV – 2% HEZJM added to the polymer; GROUP V - control group; GROUP VI – Polymer replaced by 1% ketoconazole; GROUP VII – polymer replaced by 2% ketoconazole; GROUP VIII – 1% ketoconazole added to the polymer; GROUP IX – 2% ketoconazole added to the polymer. The specimens were placed on plates containing Sabouraud agar with Candida albicans. Plates were then kept in a bacteriological incubator at a temperature of 37°C for 48 hours. In groups I and II, specimens were not colonized. In groups III and IV, there was no inhibition zone or adherence of colonies. In group V, the surfaces of five of the ten specimens were colonized. In group VI, all specimens showed fungistatic action. Group VII presented three specimens that formed halos of inhibition and four specimens that altered the growth pattern of the colonies. Group VIII altered the growth pattern of two specimens. In group IX, colonization of the specimens did not occur. The authors concluded that in the tested specimens there was no colonization of the surface, and replacing the polymer with 2% ketoconazole showed the best results.
2.Introduction
3.Materials and Methods
3.1 Obtaining the hydroalcoholic extract of Ziziphus Joazeiromartius (HEZJM)
3.2 Obtaining the molds
3.3 Obtaining the test specimens
3.4 Application of test specimens in fungal culture
3.5 Materials and Methods
4.Results
4.1 Groups I and II
4.2 Groups III and IV
4.3 Group V
4.4 Groups VI and VII
4.5 Groups VIII and IX
5.Discussion
6.Conclusion
7.Acknowledgements
8.References
Keywords
Ziziphus joazeiro Martius; Ketoconazole; Denture Stomatitis.
Introduction
Denture-related stomatitis is an inflammatory process of the oral mucosa [1-5]. Esthetic concerns often mean that patients use their dental prosthesis continuously, which can trigger inflammatory changes in the mucosa, particularly in the contact area at the base of the prosthesis. These patients often complain of a burning sensation, discomfort, or a bad taste [4, 6]. The etiology appears to be multifactorial and mainly affects patients who have an impaired immune system, advanced age, systemic disease, poor oral hygiene, a reduction in salivary flow, and in smokers and drinkers. Other factors including using dentures at night, denture trauma, the denture base material, the age of the dentures, the pH of the denture plaque, and dietary factors [2, 3, 6]. Despite these predisposing factors, the presence of Candida spp. in the biofilm of the prosthesis is considered the most important factorfor the development of this type of inflammation [2, 4, 6, 7]. Additionally, Candida-associated denture stomatitis is a commonly recurring disease, observed in approximately 11% to 67% of otherwise healthy denture wearers [3, 4, 6].
Clinically, denture-associated stomatitis presentation is variable and may be associated with chronic erythema located in the tissues under the prosthesis, sometimes accompanied by hemorrhagic petechiae, with a velvety to a rough aspect, and can present as generalized inflammation with papillary hyperplasia and lesions localized on the palate and alveolar ridge [2, 5]. Treatment involves a three-way approach: denture hygiene, correction of denture faults, and/or medication [4]. This is necessary because the prosthesis has irregular and porous surfaces in which Candida spp. adheres easily; brushing cannot remove them. The incorporation of an antifungal agent as a coating on the prosthesis has been recommended for patients with prostheses who have trouble keeping an antifungal agent in the mouth for a few minutes [8]. Several antifungal agents are being developed for the treatment of oral candidiasis, each with its advantages and disadvantages [9]. However, the toxicity and resistance of these antifungals are problems that have led to variable results and a high rate of recurrence. Some natural products have been shown to be an alternative to synthetic chemicals [3, 6]. Furthermore, these medicinal plants can play a very important role in the treatment of denture stomatitis [3], because acrylic resins are prone to microbial adherence, especially by Candida albicans. Surface-charged resins alter the ionic interaction between the denture resin and Candida hyphae, and these resins are being developed as a means to reduce microbial colonization on the denture surface [10].
C. albicans has been shown to be associated with denture-related stomatitis [2, 6], and taking into account that the surface of the acrylic resin used for dentures is prone to microbial adherence, especially C. albicans [10], and the display boards of this resin to oral fluids enhances this condition of adhesion, as demonstrated in vitro by Samaranayake et al., [11] this study aimed to investigate changes in the resin composition used for prostheses to impair microbial adherence, because the role of candidal colonization of the oral cavity and dental prostheses in complete denture wearers is not clear in the literature [1]. Therefore, the aim of this study was evaluate, in vitro, the antifungal effect of a hydroalcoholic extract of Ziziphus joazeiro Martius (HEZJM) and ketoconazole in thermally activated acrylic resin used as the base of dental prostheses against C. albicans. Our hypothesis was that HEZJM and ketoconazole would have an important antifungal effect against C. albicans.
Obtaining the hydroalcoholic extract of Ziziphus Joazeiromartius (HEZJM)
HEZJM was prepared from scraping shoots from the base of a single mature tree. The chips were air-dried to prevent the growth of microorganisms, sprayed on feed. In total, 2 kg of material was obtained, which was added to 10L of 90% ethanol in a percolator (Permution, E. J. Krieger & Cia Ltda, Curitiba, PR, Brazil); this process was repeated three times. The obtained filtrate was submitted by portions to a rotary evaporator apparatus (Rotavapor R-200, Büchi, Flawil, Switzerland), and the resulting crude extract was placed in an exhaust hood to remove the ethanol residue. The product was divided into 1g portions, dried in a bacteriological incubator (Fanem Ltda, São Paulo, SP, Brazil) at 37°C for two weeks, and crushed manually. Then, it was treated using an air pump (Tecnal Ltda, Piracicaba, SP, Brazil) for 8 h to remove excess moisture. The obtained powder was kept in a bacteriological incubator at 37ºC until use. Ketoconazole powder was obtained by grinding 200 mg ketoconazole tablets (Prati-Donaduzzi, Toledo, PR, Brazil).
Obtaining the molds
The molds were 10mm in diameter and 3mm thick, obtained from wax models (Technew, Rio de Janeiro, RJ, Brazil) using type IV plaster (Pasom Gold Star Brasil Ltda, Mairiporã, SP, Brazil), using dental flask used to include dental prosthesis. The bottom of the furnace was filled with common type II plaster (Pasom Gold Star Brasil Ltda, Mairiporã, SP, Brazil), then a layer of special type IV plaster was placed and the wax models were seated in the plaster. After the plaster setting reaction, the surface was coated with Cel Lac®. After drying the Cel Lac®, the wax models attached to the base of the dental flask were coated with another portion of type IV plaster, and then the dental flask ring was seated and completely filled with ordinary type II gypsum. With the cover in position, the dental flask was pressed manually to promote their attachment and remove excess plaster. Then, the mold was brought to a dynamic press, until the gypsum was set. Then, the parts of the dental flask were separated with the aid of a knife for plaster and the exposed surface of the base was subjected to a heated water bath to plasticize the wax models, which were removed, resulting in cavities that served as templates for the specimens.
Obtaining the test specimens
Ten specimens were made for each group with a different composition. The polymer and the monomer (Clássico, Artigos Odontológicos Clássico Ltda, Campo Limpo Paulista, SP, Brazil) were weighed and measured quantities using an electronic precision balance (Bel Mark 330, BEL Engineering, Monza, Italy), determining the corresponding weight standard measures established for each group. Standard measures were 20g for the polymer and 10ml for the monomer. Then, the materials for the preparation of samples were divided into groups I-IX, as shown in Table 1.
Table 1. Characteristics of each group regarding the composition, weight of the polymer and the monomer, and test substances.
Next, 1% and 2% of the weight of the test substances were calculated and weighed on a precision electronic balance. Incorporation (replacement or addition) of the test substances was always performed to the polymer, and these, to the monomer. The mass obtained by polymerization following contact between the reagents was placed in the cast plaster dental flask, which was previously isolated with insulating to resin.
A cellophane sheet was placed over the resin and the dental flask was closed, which was then placed in a hydraulic press. The dental flask was subjected to a polymerization cycle (74°C for 9 h), which was performed as previous recommended [10]. After the polymerization was performed, the samples were finished to remove burrs and stabilize the specimens, which were then immersed in distilled water at room temperature for 48 hours in order to remove residual monomer.
Application of test specimens in fungal culture
Samples of Candida albicans were obtained from the microbiology laboratory of the Alberto Antunes University Hospital, Federal University of Alagoas (UFAL) and identified, and storage less than two weeks. The resin specimens from each group were placed in Petri dishes containing culture medium (Sabouraud Dextrose Agar, Himedia, Mumbai, India), and Candida albicans was seeded by distributing the ten specimens of the same group on the same plate. In total, nine plates keeping them in bacteriological oven at a temperature of 37°C for 48 hours.
Evaluation of the results
The evaluation of the effect of the samples on Candida albicans cultures was visually performed to see if the specimens were colonized, if specimens promoted a change in the formation and/or growth of colonies, and if inhibition halos formed.
Groups I and II
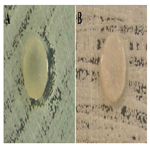
Groups I and II seeded on Candida albicans culture allowed to the formation of colonies in the culture medium without any interference. Fungicidal activity was not observed with any specimens in these two groups. The specimens were not colonized as no adherence of Candida albicans colonies was observed, indicating the fungistatic activity of the specimens for this type of fungus (Figure 1).
Figure 1. Photograph of groups I (A) and II (B) showing the growth of Candida albicans forming colonies in the culture medium.
Groups III and IV
In both groups, there was no inhibition zone around the specimens; therefore, the Candida albicans colonies showed no changes in the growth of the fungus or in its organization in colonies. Thus, the specimens did not have a fungicidal effect on Candida albicans. However, the specimens were not colonized because there was no adhesion of colonies, indicating fungistatic activity (Figure 2).
Figure 2. Photograph of groups III (A) and IV (B) showing growth of Candida albicans forming colonies in the culture medium.
Group V
The results show that no specimens in this group exerted an inhibitory effect with regard to an inhibition halo or changes in the growth pattern of Candida albicans colonies. The surfaces of fiveout ten specimens were colonized, but the remaining specimenswere free because it was found that these specimens were frostedand without translucency, which indicates the presence of a fungus such as yeast or as hyphae (Figure 3).
Figure 3. Photograph of the control group (V) showing growth of Candida albicans forming colonies in the culture medium.
Groups VI and VII
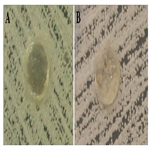
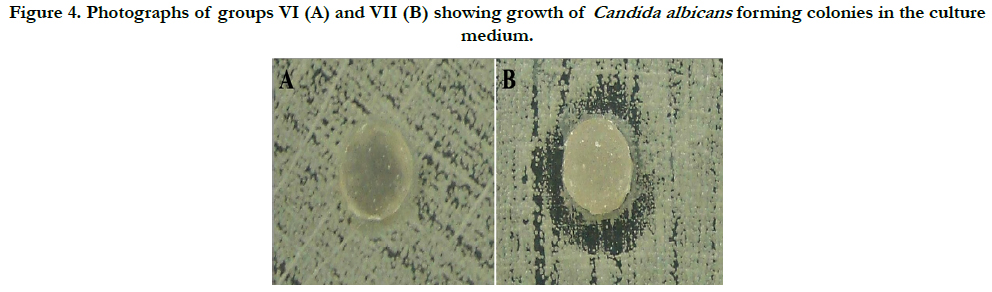
The results show that the group VI specimens had no antifungal capacity to form inhibition halos, and the remaining culture medium was fully colonized by Candida albicans. However, the surfaces of the specimens showed no adherence of microorganisms. For Group VII, the results differed from the other groups, because three specimens had antimicrobial action verified by the formation of inhibition halos. Moreover, four specimens from the same group changed in the growth pattern of Candida albicans colonies, resulting in disorganized colony growth. The fungicidal and fungistatic effects were seen in this group. The adherence of fungi on the specimens was not seen (Figure 4).
Figure 4. Photographs of groups VI (A) and VII (B) showing growth of Candida albicans forming colonies in the culture medium.
Groups VIII and IX
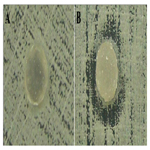
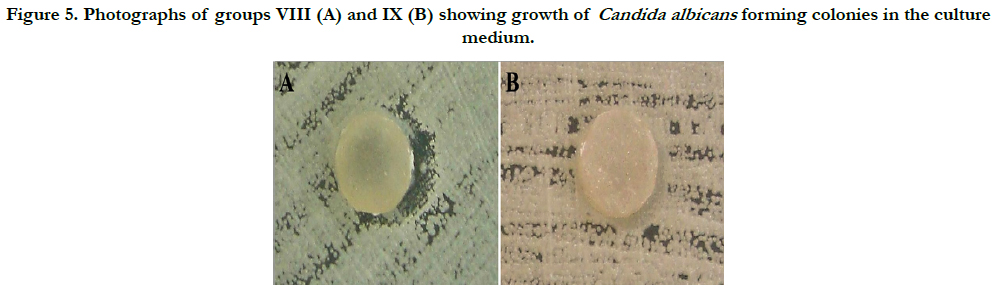
Group VIII showed a change in the growth pattern of Candida albicans colonies in two specimens. However, this effect was not observed in other specimens of the same group. In all specimens of this group, fungal adhesion was found. In group IX, the results demonstrate that Candida albicans grew and formed colonies without suffering any interference from the ten specimens. However, colonization of the specimens did not occur, indicating a fungistatic effect of the specimens (Figure 5).
Figure 5. Photographs of groups VIII (A) and IX (B) showing growth of Candida albicans forming colonies in the culture medium.
Discussion
The causes of Candida albicans infection in healthy patients are not clearly defined [12]. Under normal circumstances, factors such as diet, poor oral hygiene, changes in salivary flow rate, and systemic and localized diseases appear to facilitate the appearance of such organisms [13]. In a study conducted to demonstrate the role of Candida albicans biofilm in the etiology of denture stomatitis, it was shown that films conditioned biologically, particularly with serum, can help provide receptor binding sites for Candida albicans, which are important in initial adhesion events [14]. Additionally, Candida species usually colonize in the oral cavity of denture wearers and may also colonize their fingers because of frequent manual manipulation of the dentures. It was demonstrated that Candida species were isolated from the oral cavity of 15 (60%) and fingertips of 11 (44%) subjects, of which 10 (66.7%) subjects had concomitant oral and fingertip candidal isolation, whereas 5 (33.3%) subjects had only oral Candida. It was concluded that the hands of denture wearers who had oral Candida were significantly more colonized with Candida species than oral Candida-free subjects. The authors highlighted that patients with removable prostheses should be educated about the importance of denture and personal hygiene and the possibility of fungal hand contamination due to denture handling [1].
The results of groups I and II, which had the standard acrylic resin thermally activated modified by replacing 1% to 2% with HEJZM, respectively, showed significant antifungal activity with regard to the ability to prevent the adhesion of Candida albicans colonies to specimens in these groups. These results are similar to those obtained by Cruz et al., [15] in a study on the antifungal activity of Brazilian medicinal plants. These authors found that only extracts of Ziziphus joazeiro and Caesalpinia pyramidalis obtained by infusion in water showed significant antifungal activity against Trichophyton rubrum, Candida guilliermondii, Candida albicans, Cryptococcus neoformans and Fonsecaea pedrosoi when compared to the antifungal agent amphotericin B. The antifungal effect of a substance incorporated into the base of a dental prosthesis is important, because as demonstrated by a previous study, a higher prevalence of yeast carriers, yeast colony number, and plaque coverage have been found on the dentures of individuals with the most extensive inflammation [2]. Antifungal agents derived from plant products to treat Candida-associated denture stomatitis have been assessed by other authors. It was found that a Z. multiflora gel could reduce surface erythema of the palate more efficiently than a miconazole gel, but did not reduce the colony count on the denture surface as efficiently as miconazole [4].
In groups III and IV, the standard measure was changed by the addition of 1% and 2% of HEZJM, respectively. Antifungal activity observed with these groups, in accordance with the results of previous research showing that Ziziphus joazeiro Martius is a medicinal plant with activity against Candida albicans [15]. A study by Cruz et al., [15] showed that an extract of Ziziphus joazeiro had significant antifungal activity and properties that support its use in treating some mycoses, corroborating the importance of ethnopharmacological surveys in selecting plants for screening for bioactivity. The results of these four groups combined provide direction on the procedure to obtain HEJZM and indicate its antifungal activity when added to thermally activatedacrylicresin resin. The antifungal activity found in these groups highlighted the need for the development of a prosthesis base with continuous antifungal activity, because the presence of C. albicans, as well as cohabitation of different Candida species, is frequent in denturerelated stomatitis [2]. Additionally, a Brazilian commercial ethanol propolis extract, formulated to ensure physical and chemical stability, was found to inhibit oral candidiasis in 12 denturebearing patients with prosthesis stomatitis candidiasis [16].
In group V, which was the control group in this study, 50% of the specimens were colonized by Candida albicans. This result demonstrates that this type of fungus has the ability to adhere to the surface of the acrylic resin. These findings are consistent with earlier publications, which agree that Candida albicans is able to adhere to acrylic dental prostheses, which is the first step in the infection process. This eventually results in different degrees of denture stomatitis in the adjacent mucosa [14,17].
Ketoconazole was the first broad-spectrum imidazole administered orally and is effective against many fungal infections previously treated by amphotericin B. It is well-absorbed undernormal gastric conditions. Ketoconazole, 200 to 400 mg/day, is used to treat chronic mucocutaneous candidiasis, and is also used to treat oral, esophageal, and vaginal candidiasis [18]. However, some antifungal agents can present resistance to C. albicans, as a previous study demonstrated that C. albicans biofilms are intrinsically resistant to fluconazole, and the activity of this azole derivative against biofilms is reduced up to 4000 times compared with its activity against planktonic cultures. Amphotericin B has some activity against C. albicans biofilms [6].
Groups VI and VII incorporated ketoconazole as the test substance by replacing 1% or 2% of the standard measure. These specimens showed an inhibitory effect, especially those in group VII because three out ten of these specimens showed inhibition halos, and four out ten modified the growth pattern of Candida albicans colonies. In a study investigating different antifungal agents, the results were also satisfactory because fungal growth could be inhibited by 50% to 60% and 70% to 80% for amphotericin B and nystatin, respectively [19]. This inhibitory activity against C. albicans is relevant because a total of eight different Candida species have been isolated from the mouth, and Candida albicansis the most frequently isolated species in the mouth [1]. Another study evaluated the efficacy of miconazole 2% gel and imidazole compared to that of a Zataria multiflora 0.1% gel (a derivative of a plant with antifungal activity) found that miconazole treatment reduced the number of denture colonies more efficiently than Z. multiflora [4].
The results of groups VIII and IX, which had ketoconazole added to the standard measure in percentages of 1% and 2%, respectively, showed satisfactory results because both inhibited the adhesion and formation of Candida albicans colonies. These results are consistent with research performed by Kutzer et al., [20] which showed that C. albicans is sensitive to fluconazole and ketoconazole. From a clinical point of view, these results support the incorporation of an antifungal agent into the prosthesis base to prevent denture-related stomatitis. It is important to note that a greater extent of affected tissue means a higher likelihood that yeast is present in the subject; the number of yeasts on dentures and plaque coverage are important in denture-related stomatitis, mainly because an analysis of different stomatitis categories showed that the presence of yeast on dentures is significantly associated with the extent of inflammation [2]. The incorporation of other antifungal agents in the acrylic resin is supported by the results of the present study. Additionally, Redding et al., [19] in a previous study, with a thin-film polymer coating alone, without an antifungal agent, led to a small but significant reduction in C. albicans biofilm formation compared with control. Specifically, incorporation of antifungal medications into a thin-film polymer reduced biofilm formation between 70% and 80% with nystatin, and between 50% and 60% with amphotericin B. Biofilm reduction with chlorhexidine (up to 98%) was significantly greater than all other formulations tested. The authors concluded that an ovel thin-film coating with various antifungals effectively inhibits C. albicans biofilm formation and should be evaluated as a potential preventive therapy for denture stomatitis [19].
The positive results obtained in this study in relation to the inhibition of Candida species are important to wearers of dental prostheses because wearing a dental prosthesis such as a removable denture or removable orthodontic appliance is known to increase oral candidal colonization and to predispose the wearer to oral candidosis [1]. Additionally, the literature has shown that Candida species have a high affinity for the outer surface of dentures, and subsequently colonize the denture acrylic resin material [11]. Subsequently, when removable denture wearers use their fingers to take their prosthesis out of their mouth, either to cleanse or simply check it, oral Candida may contaminate the wearers’ fingers [1]. The incorporation of an antifungal agent in the base of a dental prosthesis can have a good effect against C. albicans, but this species can also present resistance to antifungal agents. Susceptibility testing has indicated that the biofilms produced by C. albicans have a propensity to adhere along cracks and imperfections in the denture acrylic, and additionally show increased resistance to antifungal treatment [6]. Further studies are needed to establish the efficacy of higher concentrations of HEJZM and to establish the possible influence on the properties of acrylic resin in the short, medium, and long term. The antifungal activity of medicinal plants has already been investigated extensively [3, 4, 15, 21].
Conclusion
This study demonstrates that the tested substances possess antifungal activity when incorporated into thermally activated acrylic resin used for a dental prosthesis base. However, only specimens in group VII presented a fungicidal effect, forming halos of inhibition. HEJZM and ketoconazole prevented colonization of the resin surface in all tested groups, showing fungicidal and fungistatic/fungicidal effects, respectively. There was no adhesion of Candida albicans on the surfaces of the tested specimens.
Acknowledgements
The authors would like to acknowledge the Federal University of Alagoas for supporting this research.
References
- Darwazeh AM, Al-Refai S, Al-Mojaiwel S. Isolation of Candida species from the oral cavity and fingertips of complete denture wearers. J Prosthet Dent. 2001 Oct; 86(4):420-3.PubMed PMID: 11677537.
- Barbeau J, Seguin J, Goulet JP, de Koninck L, Avon SL, Rompré P, et al., Reassessing the presence of Candida albicans in denture-related stomatitis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2003 Jan; 95(1):51-9. PubMed PMID: 12539027.
- Casaroto AR, Lara VS . Phytomedicines for Candida-associated denture stomatitis. Fitoterapia. Fitoterapia. 2010 Jul; 81(5):323-8.PubMed PMID: 20026192.
- Amanlou M, Beitollahi JM, Abdollahzadeh S, Tohidast-Ekrad Z. Miconazole gel compared with Zataria multiflora Boiss. gel in the treatment of denture stomatitis. Phytother Res. 2006 Nov; 20(11):966-9. PubMed PMID: 16906641.
- Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002 Aug; 78(922):455-9. PubMed PMID: 12185216.
- Ramage G, Tomsett K, Wickes BL, Lopez-Ribot JL, Redding SW. Denture stomatitis: a role for Candida biofilms. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2004 Jul; 98(1):53-9. . PubMed PMID: 15243471.
- Radford DR, Challacombe SJ, Walter JD. Denture plaque and adherence of Candida albicans to denture-base materials in vivo and in vitro. Crit Rev Oral Biol Med. 1999; 10(1):99-116. PubMed PMID: 10759429.
- Stafford GD, Arendorf T, Huggett R. The effect of overnight drying and water immersion on candidal colonization and properties of complete dentures. J Dent. 1986 Apr; 14(2):52-6. PubMed PMID: 3469239.
- Sheehan DJ, Hitchcock CA, Sibley CM. Current and emerging azole antifungal agents. Clin Microbiol Rev. 1999 Jan; 12(1):40-79. PubMed PMID: 9880474.
- Puri G, Berzins DW, Dhuru VB, Raj PA, Rambhia SK, Dhir G, et al., Effect of phosphate group addition on the properties of denture base resins. J Prosthet Dent. 2008 Oct; 100(4):302-8. PubMed PMID: 18922259.
- Samaranayake LP, McCourtie J, MacFarlane TW. Factors affecting th in-vitro adherence of Candida albicans to acrylic surfaces. Arch Oral Biol. 1980; 25(8-9):611-5. PubMed PMID: 7023438.
- Stenderup A. Oral mycology. Acta Odontol Scand. 1990 Feb; 48(1):3-10. PubMed PMID: 2181808.
- Moreira D, Spolidorio DM, Rodrigues JA, Boriollo MF, Pereira CV, Rosa EA, et al., Candida spp. biotypes in the oral cavity of school children from different socioeconomic categories in Piracicaba-SP, Brazil. Pesqui Odontol Bras. 2001 Jul-Sep; 15(3):187-95. PubMed PMID: 11705265.
- Ramage G, Vandewalle K, Wickes BL, Lopez-Ribot JL. Characteristics of biofilm formation by Candida albicans. Rev Iberoam Micol. Rev Iberoam Micol. 2001 Dec; 18(4):163-70. PubMed PMID: 15496122.
- Cruz MC, Santos PO, Barbosa AM, Jr., de Melo DL, Alviano CS, Antoniolli AR, et al., Antifungal activity of Brazilian medicinal plants involved in popular treatment of mycoses. J Ethnopharmacol. 2007 May 4; 111(2):409-12. Epub 2006 Dec 12. PubMed PMID: 17234376.
- Santos VR, Pimenta FJ, Aguiar MC, do Carmo MA, Naves MD, Mesquita RA.Oral candidiasis treatment with Brazilian ethanol propolis extract. Phytother Res. 2005 Jul; 19(7):652-4. PubMed PMID: 16161031.
- Cannon RD, Chaffin WL. Oral colonization by Candida albicans. Crit Rev Oral Biol Med. 1999; 10(3):359-83. PubMed PMID: 10759414.
- Gupta AK, Sauder DN, Shear NH. Antifungal agents: an overview. J Am Acad Dermatol. 1994 May; 30(5 Pt 1):677-98; quiz 698-700. PubMed PMID: 8176006.
- Redding S, Bhatt B, Rawls HR, Siegel G, Scott K, Lopez-Ribot J. Inhibition of Candida albicans biofilm formation on denture material. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009 May; 107(5):669-72. PubMed PMID: 19426921.
- Kutzer E, Oittner R, Leodolter S, Brammer KW (1988) A comparison of fluconazole and ketoconazole in the oral treatment of vaginal candidiasis; report of a double-blind multicentre trial. Eur J Obstet Gynecol Reprod Biol. 1988 Dec; 29(4):305-13. PubMed PMID: 2852609.
- Santos VR, Gomes RT, de Mesquita RA, de Moura MD, Franca EC, de Aguiar EG, et al, Efficacy of Brazilian propolis gel for the management of denture stomatitis: a pilot study. Phytother Res. 2008 Nov; 22(11):1544-7. PubMed PMID: 18696746.