Immune Response Profiles During Asthma Attacks and Intercritical Periods In Children
Lima JS1, Santos RS2, Ribeiro BM3, Maria HC Silva4, Rodrigues V5*
1 M.S. in Clinical Pathology, Graduate Program in Health Sciences, Federal University of the TriânguloMineiro (Universidade Federal do TriânguloMineiro - UFTM). Pediatrician at the Clinics Hospital of UFTM, Uberaba, State of Minas Gerais - MG, Brazil.
2 Nursing graduate, Pulmonary Function Laboratory, UFTM.
3 PhD in Clinical Pathology, Immunology Laboratory, UFTM, Uberaba, MG, Brazil.
4 PhD in Pulmonology, Pulmonology Division, UFTM.
5Full Professor of Immunology, UFTM, Uberaba, MG, Brazil.
*Corresponding Author
Virmondes Rodrigues,
Full Professor of Immunology Laboratory of Immunology,
Biological Science Institute, Universidade Federal do Triângulo Mineiro
Avenida Frei Paulino, 30, Uberaba, MG. CEP- 38125-180, Brazil.
Tel: +553433185299,
E-mail: vrodrigues@mednet.com
Article Type: Case study
Received: April 12, 2015; Accepted: July 06, 2015; Published: July 10, 2015
Citation: Lima JS, Santos RS, Ribeiro BM, Maria HC Silva, Rodrigues V (2015) Immune Response Profiles During Asthma Attacks and IntercriticalPeriods In Children. Int J Clin Med Allergy. 03(3), 26-33. doi: dx.doi.org/10.19070/2332-2799-150007.
Copyright: Rodrigues V© 2015. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Asthma is a serious public health problem due to its socioeconomic impact worldwide and affects over three hundred million individuals. This chronic inflammatory airway disease causes hyperresponsiveness, obstruction, increased mucus production and airway remodeling. Due to research advances, our understanding of the pathophysiology of asthma has changed, as has our knowledge of the cells and mediators involved in the inflammation and remodeling that result from asthma exacerbations.
The acknowledgement that asthma is much more than an inflammatory disorder that involves the T helper (Th)1-Th2 dichotomy led us to conduct the present study, which aims to determine the attack x intercritical period profile in patients with asthma. The study determined the cytokine (interleukin [IL] 1β, IL-4, IL-5, IL-9, IL-10, IL-13, IL-15, IL-17 and interferon-gamma [IFN-γ]), basophil, eosinophil and immunoglobulin (Ig) E and IgG4 profile in 30 children with asthma during attacks and in the intercritical period. The correlations between Th1 and Th2 and between Th17 and regulatory T cells (Treg) were analyzed, and no significant correlations were observed.
We observed higher IL-15 and IFNγ levels in patients with asthma than in controls, and no significant differences in IL-17 levels were observed when children with asthma and controls were compared.
2.Introduction
3.Materials and Methods
3.1.Sample
3.2.Clinical evaluation and blood collection
3.3.Spirometry
3.4.ELISA quantification of serum cytokines,IgE and IgG4
3.5.Statistical analysis
4.Results
5.Discussion
6.References
Keywords
Asthma; Asthma attack-intercritical period; Inflammatory mediators.
Introduction
Asthma is a chronic inflammatory airway disease that affects children and adults of all ages and ethnic groups. The disease has a variable progression, interspersing symptomatic periods, termed asthma attacks, with asymptomatic periods, also termed intercritical periods. In many individuals, asthma may have long asymptomatic periods with brief and occasional bouts of shortness of breath. Other individuals present with almost continuous coughing and wheezing and may have more severe attacks. These attacks can be triggered by viral infections, efforts or exposure to allergens or irritants [1].
When not controlled, asthma can lead to death or interfere with normal activities, seriously affecting quality of life [2]. The Word Health Organization estimates that approximately 235 million people are affected by asthma worldwide, and this number has increased in the last three decades, in both developed and developing countries [3]. Asthma is the most common chronic disease in children, exhibits a global prevalence of 11.7 to 14.1% [4], and is one of the most frequent causes of preventable hospital admissions in this age group [5].
Asthma can be diagnosed based on patient symptoms and history. The clinical diagnosis of the disease is suggested in the presence of dyspnea, chronic coughing, wheezing and chest tightness or discomfort, especially at night or early in the morning. The variability of symptoms and their onset in response to nonspecific irritants (such as fumes, strong odors and exercise) or allergens, such as mites and fungi, are manifestations that strongly suggest a clinical diagnosis of asthma. The worsening of symptoms at night and improvement after the use of specific drugs reinforces such clinical suspicions. The diagnosis can be confirmed based on tests that evaluate pulmonary function, including spirometry (before and after the use of a bronchodilator), bronchial provocation tests and serial measurements of peak expiratory flow rate [1, 6].
Classically, asthma is associated with the T helper 2 (Th2) profile. Th2 cytokines promote an increase in the production of the immunoglobulin (Ig)E antibody isotype in B cells (interleukin [IL] 4 and IL-13), the differentiation and maturation of mast cells (IL- 3, IL-9 and IL-13), the maturation and survival of eosinophils (IL-3, IL-5 and granulocyte-macrophage colony-stimulating factor [GM-CSF]), and the recruitment of basophils (IL-3 and GMCSF) [7]. However, the perception that strategies that are designed to suppress the Th2 function are not effective in treating all patients with asthma suggests that alternative immune pathways are also involved in the inflammatory response in this disease [8, 9]. The relationship between Th1 cell-mediated immunity and allergic asthma is more controversial. Conceptually, interferon-gamma (IFN-γ) is described as an antagonist mediator of the Th2 immune response. However, some studies have demonstrated that the IFN-γ secreted by CD4+ or CD8+ lymphocytes might act simultaneously with Th2 cytokines (IL-4, IL-5 and IL-13), thereby worsening allergic inflammatory responses and affecting chronicity [10, 11].
Recently, it has been shown that Th17, Th9 and Treg lymphocytes might be implicated on asthma. Increased levels of IL-17 and its mRNA have been found in the serum and airways of patients with asthma [12]. Several studies have shown that IL-17 is especially important in patients with severe forms of the disease who do not respond to glucocorticoid therapy [13].
Regulatory T cells (Treg) play an essential role in the regulation of the Th response and are characterized by their ability to produce the regulatory cytokines IL-10 and TGF-β. Among the various subpopulations of T regulatory cells [13], the CD4+CD25+ subpopulation is the most likely to suppress the allergic response [14].
Although the immune mechanisms involved in asthma are being studied due to our knowledge of the Th1-Th2 dichotomy, no consensus has yet been reached regarding the real relationship of each immune response profile with the development of asthma [14]. Furthermore, the immune response characteristics during attack and intercritical periods of patients with asthma, especially children, have not been studied extensively; therefore, these characteristics are imperfectly understood. In the present study, we correlate clinical and laboratory data and pulmonary function with markers for the Th1, Th2, Th9, Th17 and Treg immune response during attacks and the intercritical period in children with asthma.
Materials and Methods
Sample
Thirty patients with asthma aged between 5 and 12 years were evaluated and treated at the emergency units of the Children’s Hospital (Hospital da Criança) and the Clinics Hospital of the Federal University of the TriânguloMineiro (Hospital de Clínicas da Universidade Federal do TriânguloMineiro - HC-UFTM) or at the asthma outpatient clinic of the University of Uberaba (Universidade de Uberaba - Uniube) in the city of Uberaba, Minas Gerais, Brazil. Children older than 15 years or younger than 2 years, children of any age who were using corticosteroids for 5 days or more, and children who were immune suppressed were excluded from the study. Eighteen healthy children, who were attending the Children’s Hospital for routine consultations, were included in the study as a control group. These children had no history of bronchospasm and were in the same age bracket as the study group. This study was approved by ERB of UFTM.
Clinical evaluation and blood collection
The children were evaluated twice: during the asthma attack and during the intercritical period. All patients were subjected to a clinical examination, and in both evaluations, 5 mL of peripheral blood was collected in an EDTA vacuum blood collection tube to perform a complete blood count. In addition, 5 mL of peripheral blood was collected in a dry tube to measure serum cytokine levels.
During the first evaluation, a questionnaire adapted from the International Study for Asthma and Allergy in Children (ISAAC) [1] was used to obtain clinical data for use in classifying the condition as either intermittent, mild persistent, moderate persistent or severe persistent asthma. This classification was performed according to the criteria established in the IV Brazilian Guidelines for Asthma Management (IV DiretrizesBrasileiras para o Manejo da Asma) of 2006 and was based on clinical data and spirometry test results [15].
Spirometry
The spirometry test was performed during the intercritical period. The results were analyzed according to the guidelines for pulmonary function tests prepared by the Brazilian Society of Pulmonology and Phthisiology (SociedadeBrasileira de Pneumologia e Tisiologia) [16]. Airway obstruction was characterized by decreased final expiratory volume (FEV1), forced vital capacity (FVC) (< 80% of the predicted value) or FEV1/FVC ratio (< 80% of the predicted value). Obstructive disorder was classified as mild when FEV1 or FVC was between 60 and 80% of the predicted value, or when the FEV1/FVC ratio was between 60 and 80% of the predicted value; as moderate, when FEV1 or the FEV1/FVC ratio was between 40 and 60% of the predicted value, or when FVC was between 50 and 60% of the predicted value; and as severe when FEV1 or the FEV1/FVC ratio was less than 40% of the predicted value or when FVC was < 50% of the predicted value. The spirometry test was repeated after using a bronchodilator, and the response to the bronchodilator was considered positive when, after using the bronchodilator, FEV1 was ≥ 200 mL (when there was an obstruction in baseline conditions), ≥ 300 mL (when the baseline spirometry was normal), > 12% of its baseline value or > 7% of its predicted value.
ELISA quantification of serum cytokines,IgE and IgG4
IgE, IgG4 antibodies, IL1-β, IL-4, IL-5, IL-9, IL-10, IL-13, IL- 15, IL-17 and IFN-γ cytokines were quantified using ELISA. High-affinity 96-well plates (NUNC, Denmark) were coated with monoclonal antibodies that were specific for each cytokine or antibodies (eBIOSCIENCES) under conditions recommended by the manufacturer in a carbonate/bicarbonate buffer (pH 9.5) after the plates were discarded, and the plates were blocked with phosphate buffer containing 2% of bovine serum albumin (PBS-BSA) (SIGMA-USA) . Subsequently, the PBS-BSA was discarded, and the serum samples were added to columns 1 to 10 and diluted 1:2 for cytokines and 1:1000 and 1:10 for IgG4 and IgE respectively. Serial dilutions of the recombinant cytokines and IgE and IgG4 were used to prepare a standard curve.
Finally, plates were incubated with biotin specific antibodies and streptavidin peroxidase, 100 μL of developing buffer containing TMB (SIGMA-USA) was added to each well. The reaction was stopped by adding of sulfuric acid; the absorbance was measured at 450 nm together with a reference at 570 nm using an automated ELISA reader (ENSPIRE - PERKIN ELMER - USA). The cytokine and antibodies concentration was determined based on a standard curve for recombinant cytokines and purified IgE and IgG4 using linear regression.
Statistical analysis
Statistical analysis was performed using Stat View software (version 4.57, Abacus Concept, Berkeley, CA, USA). Continuous variables following a normal distribution are expressed as means ± standard deviations, and variables not following a normal distribution are expressed as medians, with ranges and percentiles. For variables following a normal distribution and exhibiting homogeneity of variance, the ANOVA test was used to compare three groups. Variables not following a normal distribution or with homogeneity of variance were analyzed using the Kruskal-Wallis test to compare three groups. Post-hoc tests were conducted to evaluate pairwise differences between three groups after controlling for Type I error across the tests using the Bonferroni/Dunn test for multiple comparisons. Differences were considered statistically significant when p<0.016. The Mann-Whitney test was used for comparing two groups, and the results were considered significant when p<0.05. Correlation analyses were performed using the Spearman test, and the results were considered significant when p<0.05.
Results
In the present study, 30 children with asthma (mean age, 7.7 ± 2.6 years; range, from 5 to 12 years) were evaluated. Of these children, 20 (66.6%) were males (mean age, 7.8 ± 2.4 years), and 10 (33.3%) were females (mean age, 7.6 ± 3.0 years). All patients were evaluated twice: during an asthma attack and during the intercritical period; the interval between evaluations ranged from 36 to 188 days (mean, 71.3 days).
Eighteen healthy children (55% males and 44.4% females) were included as cytokine measurement controls (mean age, 7.3 ± 2.1 years; range, from 5 to 12 years).
Children with asthma were classified into four categories according to the criteria established by the IV Brazilian Guidelines for Asthma Management and the International Consensus on Pediatric Asthma (ICON) based on clinical data and spirometry test results [28]. Most of the evaluated children (17/30 - 56.7%) were classified as having mild persistent asthma. Five children (16.7%) had intermittent asthma, six (20.0%) had moderate persistent asthma and only two children (6.7%) had severe persistent asthma.
Regarding the pulmonary function evaluation, 15 children (50%) had normal spirometry, and 13 children (43.3%) had an obstructive respiratory disorder, which was classified as mild in 11 cases (36.7%) and moderate in two cases (6.7%). Two children did not undergo the test. Ten children (33.3%) exhibited a significant response to the test after the use of a bronchodilator.
Samples collected during asthma attacks presented significantly higher percentages of eosinophils in peripheral blood when compared to samples collected during the intercritical period and to samples obtained from the control group (p < 0.0001). The percentage of basophils did not significantly differ between the two time-points evaluated (p = 0.0881) (Figure 1).
Figure 1 – Quantification of Eosinophils and Basophils in the Peripheral Blood of Patients with Asthma.
Serum IgE levels were significantly higher in samples obtained from asthmatic children during attacks and during the intercritical period (p = 0.0014) compared to samples obtained from the controls (Figure 2A). No significant differences in the IgE levels of samples obtained from asthmatic patients were observed between the two time-points, i.e., during the asthma attack and during the intercritical period. Serum IgG4 levels were higher in asthmatic patients than in the controls, but this difference was not significant (p = 0.068) (Figure 2B). No significant differences in the IgG4 levels of samples obtained from asthmatic patients were observed between the two time-points. Furthermore, when considering the total sample, a positive and significant correlation was found between serum IgE and IgG4 levels (p = 0.0011; r = 0.4) (Figure 2C).
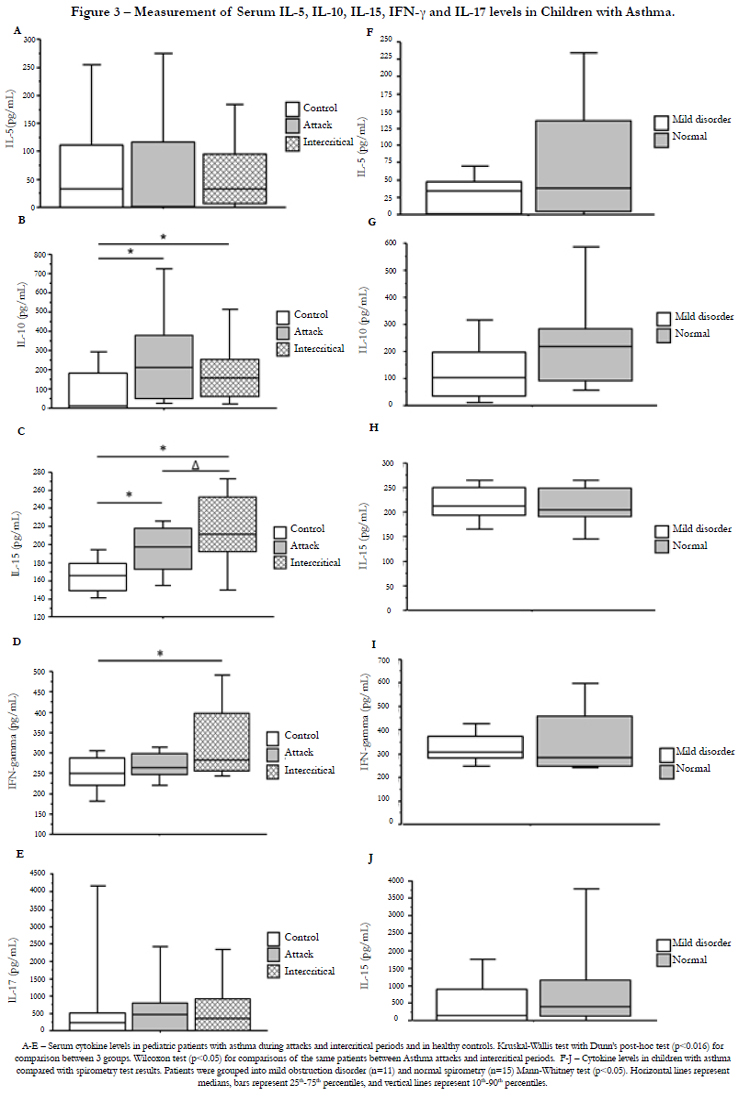
Serum IL-1β, IL-4, IL-5, IL-9, IL-10, IL-13, IL-15, IL-17 and IFN-γ levels were compared between asthmatic patients and controls and between samples collected during an asthma attack and during the intercritical period. Cytokine levels were also analyzed in asthmatic patients grouped according to the spirometry test results (Figure 3).
Serum samples obtained from asthmatic children had significantly higher IL-10 (p = 0.0042), IL-15 (p = 0.0001) and IFN-γ (p = 0.0091) levels than those obtained from controls. IL-15 levels were significantly higher during the intercritical period (p = 0.01) than during the attack period. The remaining cytokines exhibited no significant differences. No significant differences in cytokine levels were found between asthmatic patients with normal spirometry and patients with mild obstructive disorder. Because only two patients were classified as having moderate obstructive disorder, these patients were excluded from this analysis. IL-4, IL- 13, IL-9 and IL-1β were detected in very few samples. IL-4 was detected in only three (5%) of the 60 samples tested, considering those collected during both the attack and intercritical periods. Of the 18 controls evaluated, only one (5.5%) had detectable IL-4 levels. IL-13 was detected in only eight samples of asthmatic patients (13.3%) and was not detected in the controls. IL-9 was positive in four samples (6.6%) obtained from asthmatic patients and in three samples (16.6%) obtained from controls. Finally, IL-1β was detectable in only two samples (3.3%) among patients with asthma and in one sample (5.5%) among the controls. Thus, comparison of the levels of this cytokine between the groups was hindered and is therefore not shown in the figure.
To evaluate the immune response profile, the correlations between serum IFN-γ and IL-5, IL-10 and IL-5, IFN-γ and IL-10, IFN-γ and IL-17, and IL-10 and IL-17 levels were analyzed. In all studied groups, no significant correlations were found between the levels of these cytokines (Figure 4 and Table 1).
No significant correlations were found between the cytokine levels for the Th1, Th2, Th17 and Treg profiles, as shown in Figure 4 and Table 1.
Table 1 – Correlation between serum levels of IFN-γ and IL-5, IL-10 and IL-5, INF-γ and IL-10, IFN-γ and IL-17 and IL-10 and IL-17 in the studied groups.
Discussion
Asthma affects much of the world’s population at all ages and is widely distributed in both developed and developing countries. This multifactorial disease is affected by individual genetic characteristics and the environment [17]. This study correlated clinical and laboratory data, such as serum cytokine levels, in pediatric patients with asthma during attacks and intercritical periods.
Thirty children with asthma were evaluated, of whom five had intermittent asthma (16.7%), 17 had mild persistent asthma (56.7%), six had moderate persistent asthma (20.0%) and two had severe persistent asthma (6.7%). Data from Brazilian and international studies show that most cases of the disease fall into the mild persistent asthma category, as observed here [15]. This classification considers clinical aspects and pulmonary function as evaluated by the spirometry test, which provides information on the severity of airflow limitation and its reversibility after the use of bronchodilators. In the present study, of the 30 children evaluated, 15 (50%) presented normal results in the spirometry test, and 13 (43.3%) presented obstructive ventilation disorders, which were classified as mild in 11 cases (36.7%) and moderate in two cases (6.7%). Several studies suggest that the spirometry test exhibits low sensitivity in patients with mild or moderate asthma [18], which might explain the normal spirometry results that were found in half of the patients evaluated. These observations emphasize that a normal spirometry test result does not exclude the presence of asthma.
The white cell count of patients with asthma and the controls was analyzed regarding the eosinophil and basophil count. Eosinophilia is common in asthmatic patients [19], and eosinophils are an essential part of the allergic inflammation associated with asthma. The actions performed by these cells contribute to tissue damage, vascular leakage, mucus secretion and airway smooth muscle contraction [20]. In our study, asthmatic patients had a significantly higher percentage of eosinophils than the controls. Samples collected during asthma attacks also exhibited significantly higher eosinophil counts than those collected during intercritical periods, which suggests that these cells exhibit rapid mobility during events that trigger attacks.
Basophils are also involved in the Th2 response activation process, which is highly characteristic of asthma, and have been identified in airways during asthma exacerbation [21]. In the present study, no significant differences were observed between the basophil counts of the asthmatic patients and the controls. An increased number of basophils were found in the peripheral blood of asthmatic patients during attacks, but this was not significant when compared to that found during intercritical periods. These data suggest that eosinophils have more important role in asthma attacks in comparison to basophils.
IgE levels are usually increased during allergic responses, such as asthma. Thus, the determination of serum IgE levels is a common clinical practice, and the use of therapies involving anti-IgE antibodies represents an interesting alternative for the treatment of this disease]. We observed significantly higher IgE levels in children with asthma than in the controls. It suggests that IgE production is increased in asthmatic patients. This result is consistent with several studies that have reported increased IgE levels in patients with asthma [22].
The role of the immunoglobulin IgG4 is not yet fully understood. This immunoglobulin mediates immune tolerance during specific allergy immunotherapy but is also associated with tissue damage in some autoimmune diseases [23]. IgG4 has a tendency to appear only after prolonged immunization. Therefore, in the context of allergies, the appearance of IgG4 is usually associated with a decrease in symptoms [23]. In this study, IgG4 levels were higher in patients with asthma than in controls; however, this difference was not significant. The positive and significant correlation between serum IgE and IgG4 levels that was found here was also found recently in another study [24].
During asthma, several cytokines are produced, each of which has a critical role in the organization of the allergic inflammatory response [25]. However, the specific role of each cytokine that participates in this process remains unclear. Here, we measured serum IL-1β, IL-4, IL-5, IL-9, IL-10, IL-13, IL-15, IL-17 and IFN-γ levels in samples collected during asthma attacks and during intercritical periods in an attempt to better characterize cell response patterns in these patients. Of all cytokines evaluated, only IL-10, IL-15 and IFN-γ were present at significantly higher levels in children with asthma when compared with the controls. When samples collected during asthma attacks and intercritical periods were compared, only IL-5 significantly differed, presenting higher levels in samples collected during intercritical periods. IL-4, IL-13, IL-9 and IL-1β were detected in very few samples, which hindered their analysis. IL-17 was equally detected in the serum of patients with asthma and in that of the controls. Some studies on asthma reported the presence of IL-17, and this was especially associated with more severe lesions of the disease [37]. However, in our study, most patients had a mild or moderate form of the disease, and IL-17 levels were similar to those observed in the controls. A prospective study would be able to determine whether increased IL-17 levels are associated with the progression of chronic-phase lesions [36].
IL-10 levels were significantly higher in children with asthma than in the controls. Lower IL-10 levels have been found in the serum and airways of patients with asthma, and this might contribute to greater inflammation due to the lack of proinflammatory cytokine regulation [26]. IL-10 has been shown to be very important for the control of the Th2 response in airways [27]. Moreover, depending on the context of its production, IL-10 might stimulate the inflammatory response [28].
IL-10 levels are not lower in patients with mild asthma. IL-10 levels were significantly higher in the bronchoalveolar lavage fluid in patients with asthma than in the controls, both before and after allergen challenge. Furthermore, in experimental models, IL-10 -/- mice challenged with OVA were not able to develop an inflammatory response [28], which suggests that IL-10 plays a role in maintaining the asthma inflammatory response.
In our study, we also see higher IL-15 levels in patients with asthma than in the controls. IL-15 stimulates the proliferation and differentiation of T cells, NK cells, monocytes and neutrophils [29]. This cytokine uses the same receptors as IL-2; thus, they share several similar functions. IL-15 activates the production of several proinflammatory cytokines in addition to activating the production of chemokines, such as CCL5 (Rantes) [30]. This cytokine is considered to play an important role in several inflammatory and autoimmune diseases, including sarcoidosis, intestinal inflammatory diseases, multiple sclerosis and rheumatoid arthritis [31]. The results presented here reinforce findings reporting high IL-15 levels in patients with asthma [32, 36].
IFN-γ is considered an important cytokine for the pathogenesis of asthma, especially the severe form [33]. Elevated IFN-γ levels in asthmatic patients might contribute to the observed worsening of lesions and negative modulation of Th-profile cytokines (IL-4, IL-5 and IL13) [34]. Conversely, some studies have reported lower serum IFN-γ levels in children with asthma compared with controls with concomitant increases in IL-4 and IL-13 levels [35, 37].
In the present study, serum markers of inflammatory activity that are readily available in clinical practice were analyzed. The evaluation of eosinophil counts and IgE levels was helpful in understanding the events that occur during attacks and intercritical periods. Furthermore, cytokines that are involved in the modulation of inflammatory phenomena (which differ from the Th2 cytokines that are traditionally associated with asthma, such as IL-15, IL-10 and IFN-γ) were increased in the serum of children with asthma. Exploring the signaling pathways of these cytokines and their biological effects might be an important strategy to understand the development of the chronic lesions that are associated with asthma in children and their possible consequences for adults.
References
- GLOBAL-INITIATIVE-FOR-ASTHMA. Global strategy for asthma management and prevention. 2012 [cited 2014 28/12/2014]; Available from: http://www.ginasthma.org/.
- Pawankar R, Canonica GW, Holgate ST, Lockey RF (2011) WAO White Book on Allergy, Milwaukee, Wisconsin: World Allergy Organization. 230.
- World-Health-Organization. Chronic respiratory diseases. Asthma. 2014 [cited 2014 08/06/2014]; Available from: http://www.who.int/respiratory/asthma/en/.
- Mallol J, Crane J, von Mutius E, Odhiambo J, Keil U, et al. (2013) The International Study of Asthma and Allergies in Childhood (ISAAC) Phase Three: a global synthesis. Allergol Immunopathol (Madr) 41(2):73-85.
- Wallace JC, Denk CE, Kruse LK (2004) Pediatric hospitalizations for asthma: use of a linked file to separate person-level risk and readmission. Prev Chronic Dis 1(2):A07.
- Bimestral P (2012) Diretrizes da Sociedade Brasileira de Pneumologia e Tisiologia para o manejo da asma-2012. J Bras Pneumol 38(Suplemento 1).
- Ngoc PL, Gold DR, Tzianabos AO, Weiss ST, Celedon JC (2005) Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol 5(2):161-166.
- Holgate ST (2012) Innate and adaptive immune responses in asthma. Nat Med 18(5): 673-683.
- Kudo M, Ishigatsubo Y, Aoki I (2013) Pathology of asthma. Front Microbiol 4:263.
- Boniface S, Koscher V, Mamessier E, El Biaze M, Dupuy P, et al. (2003) Assessment of T lymphocyte cytokine production in induced sputum from asthmatics: a flow cytometry study. Clin Exp Allergy 33(9):1238-1243.
- Cho SH, Stanciu LA, Holgate ST, Johnston SL (2005) Increased interleukin- 4, interleukin-5, and interferon-gamma in airway CD4+ and CD8+ T cells in atopic asthma. Am J Respir Crit Care Med 171(3):224-230.
- Agache I, Ciobanu C, Agache C, Anghel M (2010) Increased serum IL-17 is an independent risk factor for severe asthma. Respir Med 104(8):1131- 1137.
- Robinson DS, Larche M, Durham SR (2004) Tregs and allergic disease. J Clin Invest 114(10):1389-1397.
- Ellwood P, Asher MI, Beasley R, Clayton TO, Stewart AW (2000) International Study of Asthma and Allergies in Childhood - Phase Three Manual, Auckland, New Zealand: ISAAC International Data Centre. 94.
- Stirbulov R, Bernd LAG, Sole D (2006) IV Diretrizes Brasileiras para o Manejo da Asma. J Bras de Pneumol 32(Suppl 7):S447- 474.
- Rodrigues JC, Cardieri JMA, Bussamra MHCDF, Nakaie CMA, Almeida MBD, et al. (2002) Provas de função pulmonar em crianças e adolescentes. J Pneumol 28(Suppl 3):S207-S221.
- Busse WW, Rosenwasser LJ (2003) Mechanisms of asthma. J Allergy Clin Immunol 111(3):S799-S804.
- Schneider A, Gindner L, Tilemann L, Schermer T, Dinant GJ, et al. (2009) Diagnostic accuracy of spirometry in primary care. BMC Pulm Med 9:31.
- Stone KD, Prussin C, Metcalfe DD (2010) IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol 125(2 Suppl 2):S73-S80.
- Liu LY, Mathur SK, Sedgwick JB, Jarjour NN, Busse WW, et al. (2006) Human airway and peripheral blood eosinophils enhance Th1 and Th2 cytokine secretion. Allergy 61(5):589-597.
- Siracusa MC, Comeau MR, Artis D (2011) New insights into basophil biology: initiators, regulators, and effectors of type 2 inflammation. Ann N Y Acad Sci 1217: 166-177.
- Holgate ST (2013) Stratified approaches to the treatment of asthma. Br J Clin Pharmacol 76(2):277-291.
- Rogosch T, Kerzel S, Dey F, Wagner JJ, Zhang Z, et al. (2014) IgG4 and IgE Transcripts in Childhood Allergic Asthma Reflect Divergent Antigen-Driven Selection. J Immunol 193(12):5801-5808.
- Piacentini GL, Guerresi S, Kantar A, Lubrano L, Olivieri F, et al. (2011) A comparison between IgE and IgG4 as markers of allergy in children: an experimental trial in a model of natural antigen avoidance. Int J Immunopathol Pharmacol 24(4): 1049-1056.
- Farahani R, Sherkat R, Hakemi MG, Eskandari N, Yazdani R (2014) Cytokines (interleukin-9, IL-17, IL-22, IL-25 and IL-33) and asthma. Adv Biomed Res 3:127.
- Hawrylowicz CM, O'Garra A (2005) Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nat Rev Immunol 5(4):271-283.
- Oh JW, Seroogy CM, Meyer EH, Akbari O, Berry G, et al. (2002) CD4 T-helper cells engineered to produce IL-10 prevent allergen-induced airway hyperreactivity and inflammation. J Allergy Clin Immunol 110(3):460-468.
- Makela MJ, Kanehiro A, Borish L, Dakhama A, Loader J, et al. (2000) IL-10 is necessary for the expression of airway hyperresponsiveness but not pulmonary inflammation after allergic sensitization. Proc Natl Acad Sci U S A 97(11):6007-6012.
- Ye J (2014) Beneficial metabolic activities of inflammatory cytokine interleukin 15 in obesity and type 2 diabetes. Front Med 1-7.
- Chenoweth MJ, Mian MF, Barra NG, Alain T, Sonenberg N, et al. (2012) IL-15 can signal via IL-15Rα, JNK, and NF-kB to drive RANTES production by myeloid cells. J Immunol 188(9):4149-4157.
- Laza-Stanca V, Message SD, Edwards MR, Parker HL, Zdrenghea MT, et al. (2011) The role of IL-15 deficiency in the pathogenesis of virus-induced asthma exacerbations. PLoS Pathog 7(7).
- Bosco A, Ehteshami S, Stern DA, Martinez FD (2010) Decreased activation of inflammatory networks during acute asthma exacerbations is associated with chronic airflow obstruction. Mucosal Immunol 3(4):399-409.
- Cho SH, Stanciu LA, Begishivili T, Bates PJ, Holgate ST, et al. (2002) Peripheral blood CD4+ and CD8+ T cell type 1 and type 2 cytokine production in atopic asthmatic and normal subjects. Clin Exp Allergy 32(3):427- 433.
- Peters MC, Mekonnen ZK, Yuan S, Bhakta NR, Woodruff PG, et al. (2014) Measures of gene expression in sputum cells can identify TH2-high and TH2-low subtypes of asthma. J Allergy Clin Immunol 133(2):388-394.
- Kumar RK, Yang M, Herbert C, Foster PS (2012) Interferon-gamma, pulmonary macrophages and airway responsiveness in asthma. Inflamm Allergy Drug Targets 11(4):292-297.
- Chakir J, Shannon J, Molet S, Fukakusa M, Elias J, et al. (2003) Airway remodeling-associated mediators in moderate to severe asthma: effect of steroids on TGF-beta, IL-11, IL-17, and type I and type III collagen expression. J Allergy Clin Immunol 111(6):1293-1298.
- Barczyk A, Pierzchala W, Caramori G, Wiaderkiewicz R, Kaminski M, et al. (2014) Decreased percentage of CD4(+)Foxp3(+)TGF-beta(+) and increased percentage of CD4(+)IL-17(+) cells in bronchoalveolar lavage of asthmatics. J Inflamm (Lond) 11:22.