Protective Effect of Montelukast against Cadmium Induced Pituitary Gland Toxicity
Abd El Azeem EK, Abass MF, Paulis MG, Nisreen Abd-eltawab Abd-Elgaber*
Forensic Medicine and Clinical Toxicology Department & Pathology Department, Faculty of Medicine, Minia University, Egypt.
*Corresponding Author
Melad G. Paulis
Assistant Professor of Forensic Medicine and Clinical Toxicology,
Faculty of Medicine, Minia University, Egypt.
Tel: 02/01222993067
E-mail: melad.boulis@mu.edu.eg
Received: February 20, 2017; Accepted: March 16, 2017; Published: March 20, 2017
Citation: Abd El Azeem EK, Abass MF, Paulis MG, Nisreen Abd-eltawab Abd-Elgaber (2017) Protective Effect of Montelukast against Cadmium Induced Pituitary Gland Toxicity. Int J Clin Pharmacol Toxicol. 6(2), 256-261. doi: http://dx.doi.org/10.19070/2167-910X-1700043
Copyright: Nisreen Abd-eltawab Abd-Elgaber© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Cadmium (Cd) is a common environmental hazardous heavy metal. The standard treatment of heavy metal intoxication; chelators may have a minor role in Cd toxicity. Montelukast (ML) is an anti-inflammatory drug that is used mainly for managing asthmatic patients. Recent workers have tested ML for its antioxidant properties in various conditions and obtained encouraging results as regarding this effect. We investigated the protective and antioxidant effect of ML on pituitary gland, a highly sensitive organ to Cd toxicity. Four groups of rats were used and received distilled water, 10mg/kg ML, 3mg/kg Cd, and 10mg ML plus 3mg/kg Cd respectively. Antioxidant, histological, immunohistochemical and hormonal effects of Cd and ML were evaluated. The detrimental effect of Cd on pituitary gland was abolished by ML. The diminished hormonal effect of Cd was restored to normal levels. The increased MDA level and depleted oxidative enzymes on pituitary gland of rat exposed to Cd were restored by ML. lastly, histological and apoptotic effects of Cd were also, corrected significantly by ML. It is concluded that ML can protect against Cd-induced pituitary gland toxicity.
2.Introduction
3.Materials and Methods
4.Immunohistochemical Staining
5.Results
6.Discussion
7.Conclusion
8.References
Keywords
CD; Montelukast; Pituitary; Antioxidant; Apoptosis.
Introduction
Cadmium (Cd) is one of the commonest toxic heavy metal in the surroundings and forms with other heavy metals one of the oldest environmental health problem [1]. It is extensively used in many industries as rechargeable batteries, pigments, radiation screens, etc. In addition, natural health and pharmaceutical productsmay be sources of Cd exposure. So, we could be exposed to this metal environmentally, occupational and by diet. Cd has serious health hazards that affect most of the tissue types of the human body including reproductive function [2] liver [3] pancreas [4], heart [5] and nervous system [6]. It has been classified as class I human carcinogenic agent [1].
Although, the evidenced high deleterious effect of Cd on the body; there is a shortage in its effective treatment. The standard management of heavy metal intoxication; chelators may have a minor role in Cd toxicity as these therapies have little ability to penetrate cells where most of Cd was stored [1, 7]. With increasing evidence of oxidative stress as a possible mechanism of Cd toxicity [8], variable antioxidants have been tried as possible protective agents in Cd toxicity for example zinc [9] selenium [10], vitamin C and E [11].
Montelukast (ML) is an anti-inflammatory drug with selective reversible cysteinyl leukotriene D4-receptor blocking effect that is used mainly for managing asthmatic patients. Recent workers have tested ML for its antioxidant properties in various conditions and obtained encouraging results as regarding this effect [12]. Most of the studies used ML for acutely intoxicated conditions without looking for subchronic or chronic cases. Also, up to our knowledge, there is no research regarding its protective effect on heavy metal toxicity. So, in this study, we investigated the protective and antioxidant effect of ML on a highly sensitive organ to Cd toxicity; pituitary gland. Hormonal, structural, and antioxidant effects were used to evaluate the toxic effect of Cd and the protective properties of ML.
Materials and Methods
Adult male Sprague rats weighting (220 ± 30gm) were purchased from Faculty of Agriculture, Minia University Egypt. Experimental procedures followed the guidelines of Minia University Ethical Committee for care and manipulation of animals used for the research. Animals were left for 1 week in the same experimental conditions with free access to food ad libitum for acclimatization. Another week was left in the same condition to calculate the water consumption of rats. Rats consumed water about 20ml/day.
Cadmium chloride was obtained from Merck®, Egypt. CdCl2 anhydrous (99.995%-Cd pure white powder, 25gm package) was prepared by dissolving 50 mg into one liter of distilled water. ML as Singulair® (5mg tablets) produced by Global Napi Pharmaceuticals (GNP) - Egypt. Animals were divided into 4 groups (10 rats each) as follow: the control group received distilled water. Group 2 received ML diluted in distilled water at a dose of 10 mg/kg/ day orally [13]. Group 3 received Cd at 3 mg CdCl2/kg/ day (50 mg/l CdCl2) (this dose produces neuroendocrinal disruption) [14]. Group 4 received both ML plus Cd at a dosage like group 2 and 3. This regimen was followed for 1 month. Rat water consumption does not change throughout the experiment. At the end of the experimental period, animals were killed by decapitation. Trunk blood was collected in tubes containing EDTA and plasma was obtained after centrifugation of the samples at 1,500×g for 15 min at 4°C. Samples were kept frozen at −20°C until hormone measurements. Pituitary gland was removed and frozen for further examination.
For oxidative stress assessment, a part of the pituitary gland was homogenized in cold sodium phosphate buffer (pH 7.4) containing 1mmol/L ethylene diamine tetra acetic acid. The homogenates were then centrifuged at 9000 g for 15 min at 4°C. The supernatants were separated and used for oxidative stress analysis. Four markers were examined to test the antioxidant activities of ML. One of the extensively used tools for evaluating oxidative stress is malondialdehyde (MDA) [15]. Thiobarbituric acid (TBA) method was used to determine MDA level according to Ohkawa et al., (1979) [16]. Three antioxidant enzymes levels were assayed; namely glutathione reductase (GSH), superoxide dismutase (SOD) and catalase (CAT) according to McGowan (1989) [17], Lu et al., (2013) [18] and Aebi (1984) [19] respectively.
Biopsies from pituitary tissue samples were dehydrated and embedded in paraffin using the routine histological procedures. Serial cross sections of 6 μm were prepared. The sections were mounted and stained with hematoxylin-eosin.
Immunohistochemical Staining
The steps and methods used in immunohistochemical staining were the same followed by Hsu et al., (1981) [20]. In brief; 6μ thickness sections of pituitary tissue and fixed by poly-1-lysine and left for 12 h at 56C. Sections were deparaffinization by Xylene. 3% H2O2 in distilled water was used for inhibiting endogenous oxygenase enzymes. Then sections were washed with phosphate- buffered saline (PBS). Blocking the nonspecific binding of antibodies was done by incubation of the sections in normal coat saline and then rinsed with PBS. Lastly; incubation of the sections with p53 antibodies overnight at 4C.
A semi-quantitative method for evaluation toxic effect of Cd and protective effect of ML is apoptotic index (AI). AI was determined by counting at least 1000 cells per slide subdivided in 10 fields chosen randomly at x400 magnification. P53 stained nucleus appears dark brown. (Xu et al., 2007)[21].
AI = (number of p53 labeled cells/total number of cells) X 100
Hormonal assay was done using the commercial radioimmunoassay kits. The data were statistically analyzed using SPSS Version 22 software. All data were expressed as means ± standard errors. Data of different groups were compared using ANOVA followed by Mann–Whitney Rank. Differences at p < 0.05 were considered significant.
Results
Table (1) summarizes the effect of Cd on pituitary hormones. Serum prolactin was diminished on rats drinking water containing Cd with high significant change when compared with control group of rats. Other pituitary hormones namely LH, FSH, and GH are also, inversely affected by Cd. It is significantly lower than that of the control. In the administration of ML with Cd, all the examined hormones showed significantly elevation to approximate the normal level, these hormones become insignificantly different from that of control rats.
As regard the oxidative stress effect of Cd, MDA level was significantly higher that of the control in the pituitary tissue. Antioxidant enzymes GRH, CAT, and SOD have significantly depleted in pituitary tissues in rats received Cd. In rats received ML with Cd, there was significantly correction of the oxidative stress produced by Cd in the previous group. This appeared from the corrected MDA and antioxidant enzymes levels (Table 2).
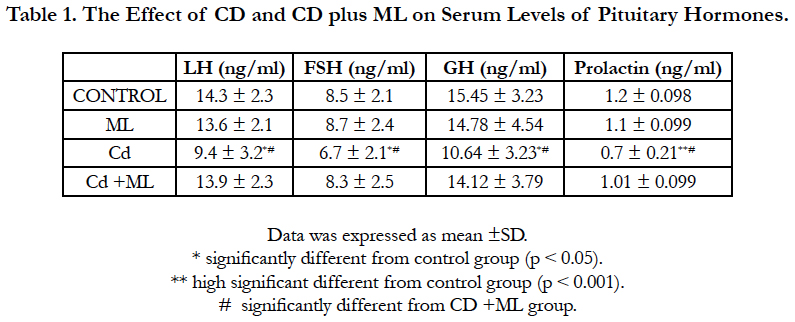
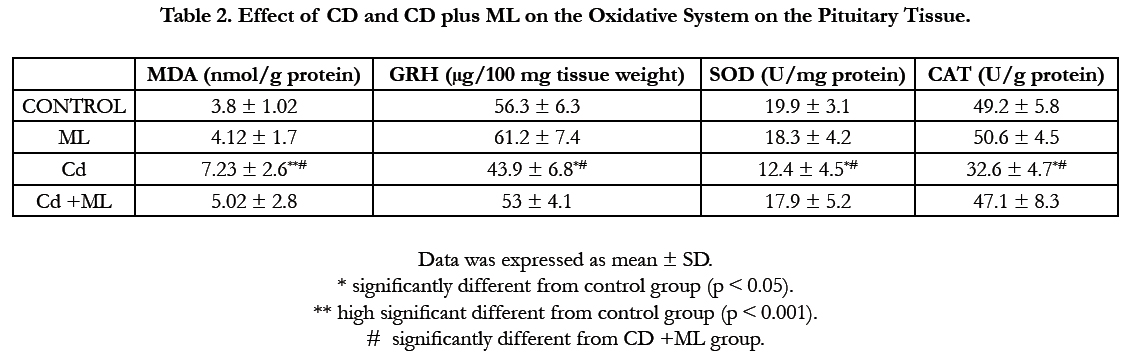
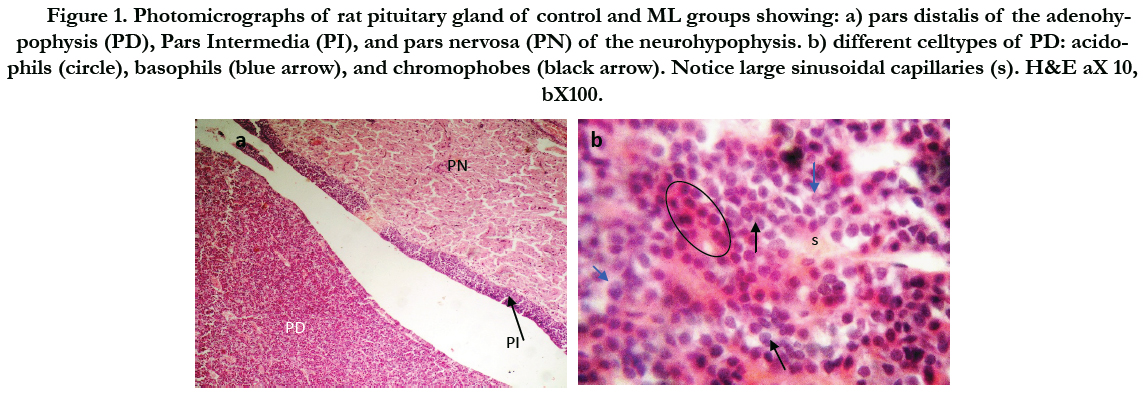
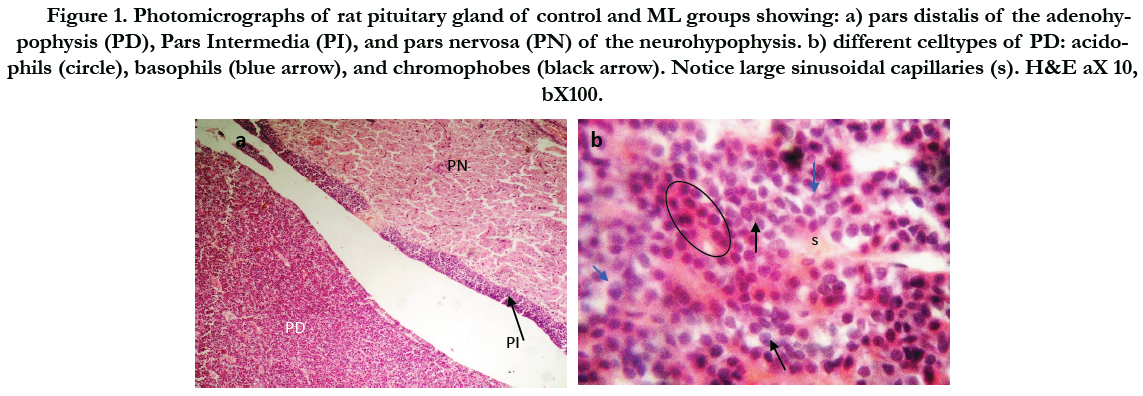
Figure (1) showed the normal histological structure of pituitary gland. Pars distalis forms the main bulk of the anterior pituitary gland. It is formed of acidophils which form the main component (secrete GH and prolactin), basophils (secrete LH, FSH, and TSH), and chromophobes. In rats received Cd, there were congested sinusoids all over the pituitary gland with decreased density of acidophils and basophils (Figure 2). In rats that were co-administered with ML plus Cd, there was an almost correction of the toxic effect of Cd on the pituitary histopathology. There was no congestion with restoring the density of the cellular elements of the anterior pituitary gland (Figure 3).
Figure 1. Photomicrographs of rat pituitary gland of control and ML groups showing: a) pars distalis of the adenohypophysis (PD), Pars Intermedia (PI), and pars nervosa (PN) of the neurohypophysis. b) different celltypes of PD: acidophils (circle), basophils (blue arrow), and chromophobes (black arrow). Notice large sinusoidal capillaries (s). H&E aX 10, bX100.
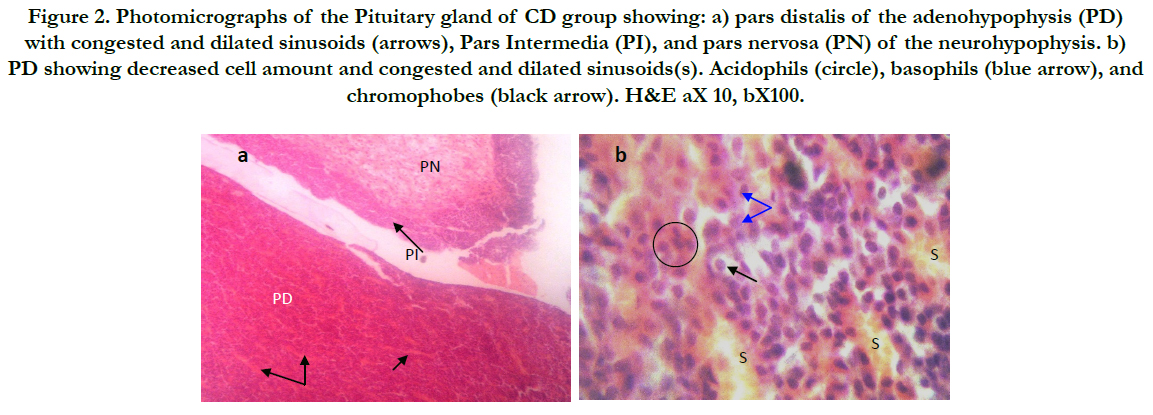
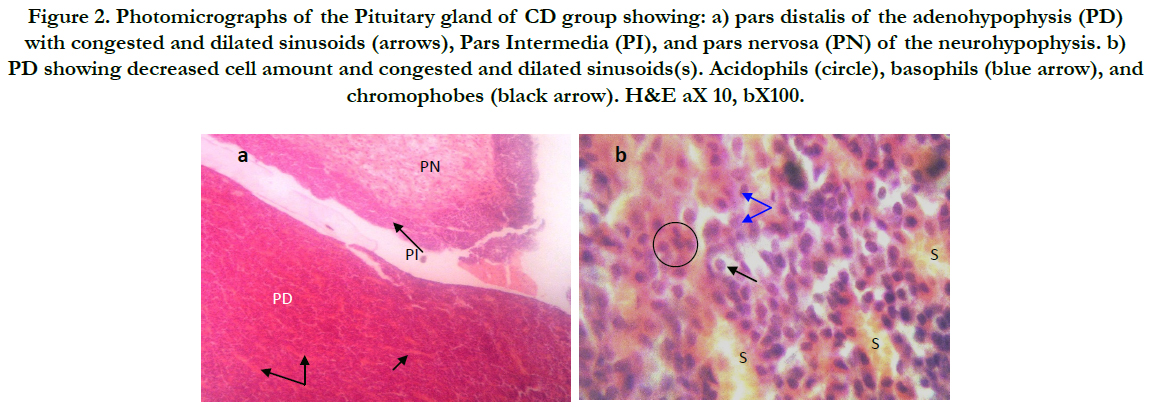
Figure 2. Photomicrographs of the Pituitary gland of CD group showing: a) pars distalis of the adenohypophysis (PD) with congested and dilated sinusoids (arrows), Pars Intermedia (PI), and pars nervosa (PN) of the neurohypophysis. b) PD showing decreased cell amount and congested and dilated sinusoids(s). Acidophils (circle), basophils (blue arrow), and chromophobes (black arrow). H&E aX 10, bX100.
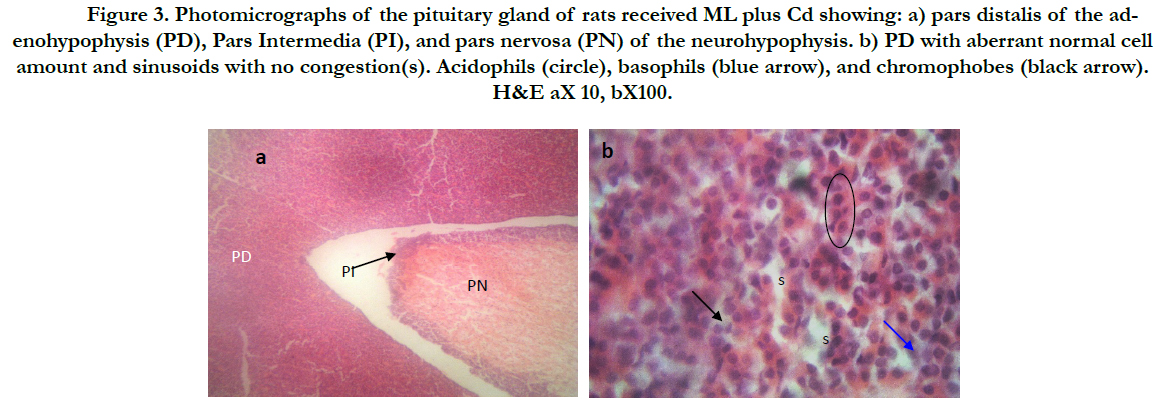
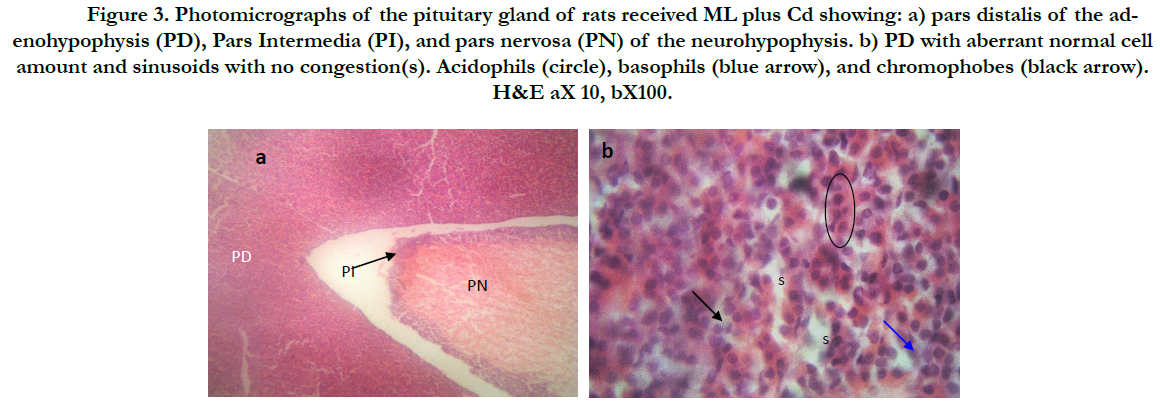
Figure 3. Photomicrographs of thepituitary gland of rats received ML plus Cd showing: a) pars distalis of the adenohypophysis (PD), Pars Intermedia (PI), and pars nervosa (PN) of the neurohypophysis. b) PD with aberrant normal cell amount and sinusoids with no congestion(s). Acidophils (circle), basophils (blue arrow), and chromophobes (black arrow). H&E aX 10, bX100.
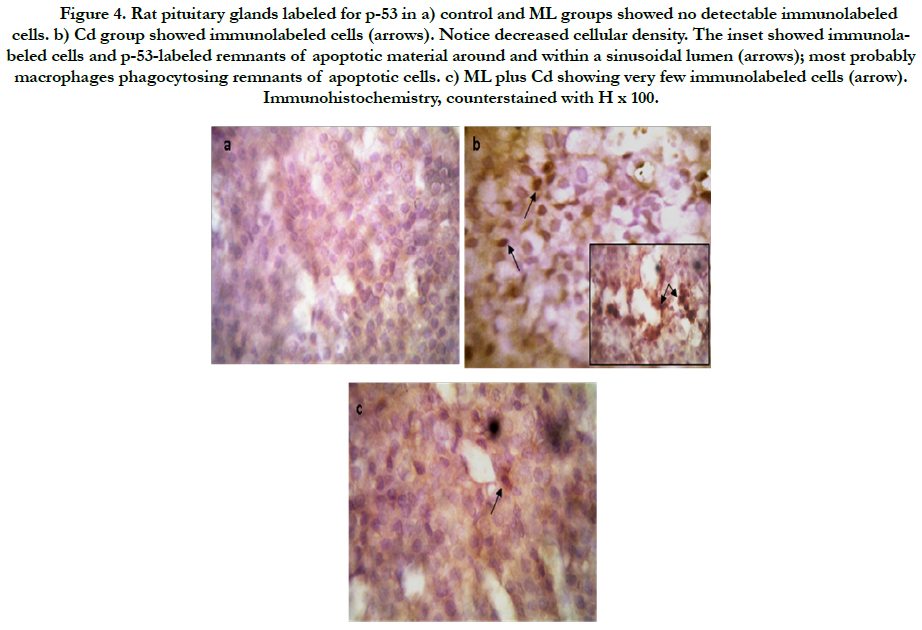
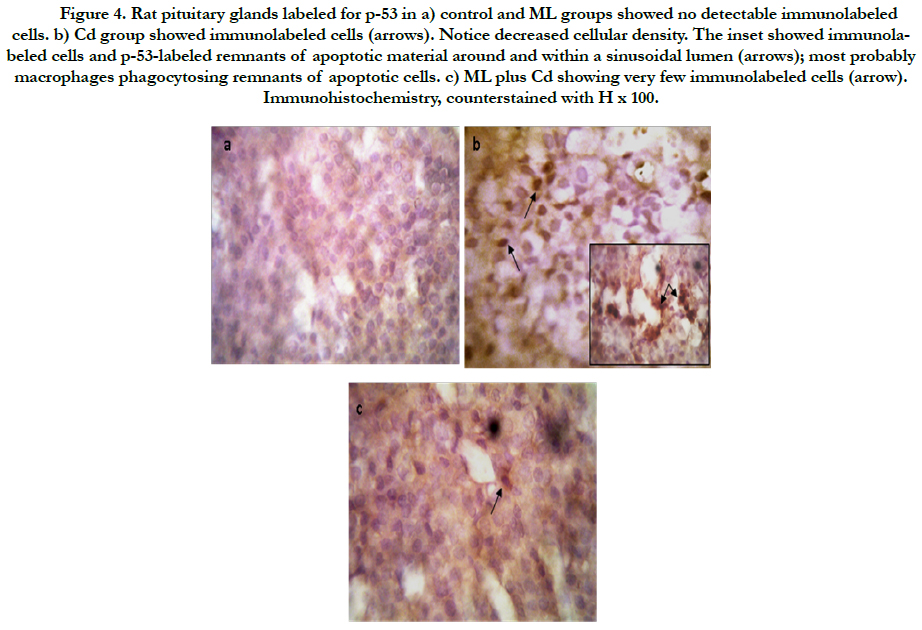
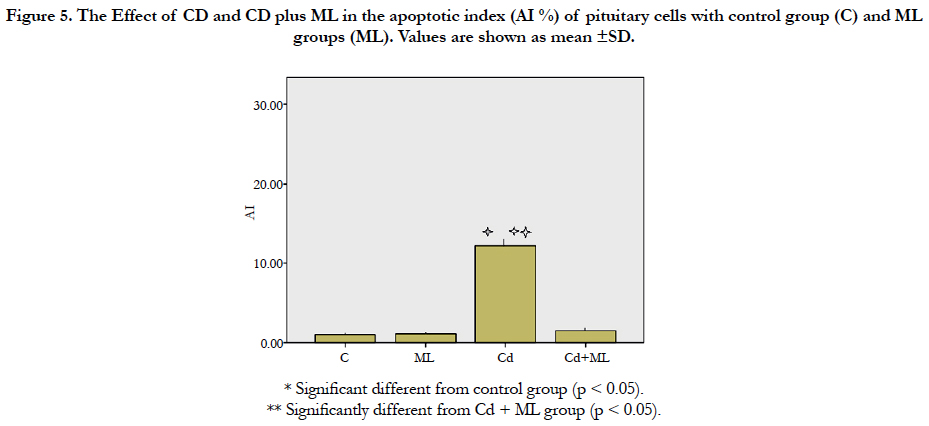
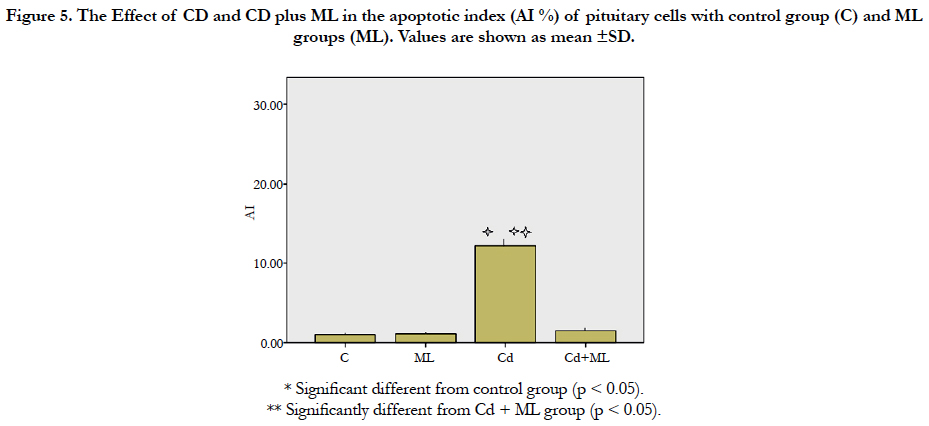
As regard immunohistochemistry, Figure (4a) shows no immunolabeled cells. Cd received rats, showed an increased number of immunolabeled cells with decreased cell density (Figure 4b). In group received ML and Cd, there were decreasing in the number of immunolabeled cells (Figure 4c). These findings reflected on the calculated AI (Figure 5). AI was significantly higher in Cd group in comparing with control group. In rats received ML plus Cd; AI is significantly decreased to nearly that of the control group.
Figure 4. Rat pituitary glands labeled for p-53 in a) control and ML groups showed no detectable immunolabeled cells. b) Cd group showed immunolabeled cells (arrows). Notice decreased cellular density. The inset showed immunolabeled cells and p-53-labeled remnants of apoptotic material around and within a sinusoidal lumen (arrows); most probably macrophages phagocytosing remnants of apoptotic cells. c) ML plus Cd showing very few immunolabeled cells (arrow). Immunohistochemistry, counterstained with H x 100.
Figure 5. The Effect of CD and CD plus ML in theapoptotic index (AI %) of pituitary cells with control group (C) and ML groups (ML). Values are shown as mean ±SD.
Discussion
Cadmium (Cd) is a ubiquitous heavy metal that is widely distributed in the environment. It is situated in the Periodic Table between zinc (Zn) and mercury (Hg) and has similar chemical characteristics as that of Zn. It usually exists as a divalent cation as CdCl2. It is widely used in industry. Smoking represents the most important source of human exposure [22]. Cd widely affects both animal and human health. It affects liver, kidney, vascular, bone, haemopoietic, and pancreatic system [3]. In addition, it has carcinogenic properties [23]. In comparison with other toxic agents, Cd occupies the 8th position in the highly toxic 20 chemicals [24]. Results of this study confirmed the detrimental effect of Cd on the pituitary hormones. It suppressed serum prolactin, LH, FSH, and GH. This was in agree with other studies in both human [4] and animal [24]. Also, Cd produced oxidative load on the pituitary gland cell. This appeared from depleted antioxidant enzymes GRH, CAT and SOD that were significantly diminished in rats received Cd. There was significant increase in lipid oxidation byproduct, MDA level. This was the same as found by different research [25, 26] in the pituitary gland and on other tissues [27-29].
Cd toxicity produced also, histological changes in rat pituitary tissues. These changes were reported in different studies [30, 31].
As regard immunohistochemical examination, there wasa significant increase in the expression of p53 protein in rat pituitary gland exposed to Cd in comparing with that of the control. This finding appeared from both microscopic examinations of immunohistologically stained sections and AI calculation. The apoptotic effect of Cd was reported by different authors on pituitary gland [32, 33] and other tissues [34-36].
Apoptosis or programmed cell death is a gene regulated mechanism that various genes control it. P53 gene is one of them. It presents at a low level in the normal cellular environment but under stressful conditions as excess reactive oxygen species and DNA damage, p53 increases to stop cell growth, repair DNA or if fail, cell death occurs [37]. There are several mechanisms involved in the apoptotic effect of Cd. Oxidative stress and disturbed calcium influx in the cell are the main mentioned causes in the literature [38]. Another mechanism was described by Tokumoto et al., and Lee et al., [39, 40]. They found that Cd suppressed gene expression of the ubiquitin-proteasome system which is responsible for the rapid destruction of this protein system and hence increased p53 concentration with subsequent cell death.
Montelukast is an oral selective leukotriene receptor antagonist that inhibits cysteine leukotriene. It is widely used as antiasthmatic therapy for chronic asthma and seasonal allergies with high tolerability in both children and adults [41, 42]. It was tested for various pathological conditions as spinal cord injury [43], hippocampal ischemia [44], and hemorrhagic shock [45]. A few years ago, it attracted scientists to be evaluated as a protection for various toxic agents. It gave good results in different acute toxicities in different organs. Examples of these agents are carbon tetra-chloride induced liver injury [46], methotrexate in kidney [47], dioxin and paracetamol in liver [12, 48], gentamicin in kidney [41], indomethacin- induced gastric ulcer [49], paraquat in lung [50], and radioiodine hepatic toxicity [13] but it is not tested against metal-induced toxicity. This study investigated if ML could protect Cd-induced pituitary toxicity. Cd is endocrinal distributors affecting all pituitary hormones also, this gland is highly sensitive to oxidative stress caused by it [25].
In rat exposed to Cd, this work found that ML could abolish all endocrinal, oxidant, and apoptotic effects of Cd. All tested pituitary hormones; prolactin, GH, LH and FSH were restored to normal levels by co-administration of Cd and ML. Pituitary gland levels of MDA, GSH, SOD, and CAT were returned to its healthy concentrations by ML. Lastly, ML succeeded in preserving the histological structure of pituitary exposed to Cd and corrected the AI.
The key feature which enabled ML to abolish Cd toxicity may be attributed to its antioxidant properties. MDA concentration in the pituitary gland which produced due to increased lipid peroxidation was restored. The antioxidant enzymes which were depleted by oxidative stress of Cd were restored by ML. the antioxidant effects of ML were reported by other authors [12, 51, 52]. ML has direct free radical scavenger ability in addition to the antiinflammatory properties which itself diminish the oxidative stress effect on tissues. The anti-inflammatory effect appeared from the disappearance of congested sinusoids in the pituitary gland of rats exposed to Cd and ML. Saad et al., stated that leukotrienes play roles in inflammation and oxidative stress [44].
ML has antiapoptotic properties. This ability was noticed from the corrected AI in pituitary tissues received Cd plus ML. This effect may be attributed to the antioxidant characteristics of ML. Several studies confirmed the fact of the ability of antioxidants to protect the cells from apoptosis [43, 53]. Another mechanism of antiapoptotic properties of ML is through inhibition of inflammation [54].
Conclusion
This is the first study to reveal the neuroprotective effect of ML on a heavy metal-induced toxicity. ML has both antioxidant and antiapoptotic protective properties against Cd deleterious effects on the pituitary gland. Further studies are needed to reveal more about the mechanism by which ML protect against Cd-induced toxicity and if it has any benefici al effect on other metals poisoning.
References
- Zhai Q, Narbad A, Chen W (2015) Dietary strategies for the treatment of cadmium and lead toxicity. Nutrients. 7(1): 552-71.
- Çilenk KT, Öztürk İ, Sönmez MF (2016) Ameliorative effect of propolis on the cadmium-induced reproductive toxicity in male albino rats. Exp Mol Pathol. 101(2): 207-13.
- Amamou F, Nemmiche S, kaouthar Meziane R, Didi A, Yazit SM, et al., (2015) Protective effect of olive oil and colocynth oil against cadmium-induced oxidative stress in the liver of Wistar rats. Food Chem Toxicol. 78: 177-84.
- Ciarrocca M, Capozzella A, Tomei F, Tomei G, Caciari T (2013) Exposure to cadmium in male urban and rural workers and effects on FSH, LH and testosterone. Chemosphere. 90(7): 2077-84.
- Sharma S, Vijaya P (2015) Cardioprotective Effects of Lycopene against Cadmium Induced Toxicity in Albino Mice. Int J Health Sci Res. 5(8): 507-13.
- Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative medicine and cellular longevity. 898034: 12.
- McCarty MF (2012) Zinc and multi-mineral supplementation should mitigate the pathogenic impact of cadmium exposure. Med hypotheses. 79(5): 642-8.
- Liu J, Qu W, Kadiiska MB (2009) Role of oxidative stress in cadmium toxicity and carcinogenesis. Toxicol appl pharmacolo. 238(3): 209-14.
- Aravind P, Prasad MNV (2003) Zinc alleviates cadmium-induced oxidative stress in Ceratophyllum demersum L.: a free floating freshwater macrophyte. Plant Physiol Biochemi. 41(4): 391-7.
- Ognjanovic B, Markovic S, Pavlovic S, Zikic R, Stajn A, et al., (2008) Effect of chronic cadmium exposure on antioxidant defense system in some tissues of rats: protective effect of selenium. Physiol Res. 57(3): 403.
- Gupta RS, Gupta ES, Dhakal BK, Thakur AR, Ahnn J (2004) Vitamin C and vitamin Е protect the rat testes from cadmium-induced reactive oxygen species. Mol cells. 17(1): 132-9.
- Bentli R, Ciftci O, Cetin A, Otlu A (2016) Anti-inflammatory Montelukast prevents toxic effects of 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin Oxidative stress, histological alterations in liver, and serum cytokine levels. Toxicol Indust Health. 32(5): 769-76.
- Atilgan HI, Yumusak N, Sadic M, Gultekin SS, Koca G, et al., (2015) Radioprotective effect of montelukast sodium against hepatic radioiodine (131I) toxicity: A histopathological investigation in the rat model. Ankara Üniv Veterinary Faculty Magzine. 62(1): 37-43.
- Caride A, Fernández-Pérez B, Cabaleiro T, Tarasco M, Esquifino AI, et al., (2010) Cadmium chronotoxicity at pituitary level: effects on plasma ACTH, GH, and TSH daily pattern. J physiol biochemi. 66(3): 213-20.
- Khoubnasabjafari M, Ansarin K, Jouyban A (2015) Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders. Bio-Impacts . 5(3): 123-7.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal biochem. 95(2): 351-8.
- McGowan C (1989) Influence of vitamin B 6 status on aspects of lead poisoning in rats. Toxicol lett. 47(1): 87-93.
- Lu X, Jin C, Yang J, Liu Q, Wu S, et al., (2013) Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats’ hippocampus. Biol trace elem res. 151(1): 75-84.
- Aebi H (1984) Catalase in vitro. Methods enzymol. 105: 121-6.
- Hsu S-M, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 29(4): 577-80.
- Xu C, Shu W-Q, Qiu Z-Q, Chen J-A, Zhao Q, et al., (2007) Protective effects of green tea polyphenols against subacute hepatotoxicity induced by microcystin-LR in mice. Envir toxicol pharmacol. 24(2): 140-8.
- Bernhoft RA (2013) Cadmium toxicity and treatment. Scientific World J. 2013: 394652.
- Ostadrahimi A, Payahoo L, Somi M, Hashemzade S, Esfahani A, et al., (2016) The association between blood cadmium levels and the risk of gastrointestinal cancer in Tabriz, northwest of Iran. Polish Ann Med.
- Jiménez-Ortega V, Barquilla PC, Fernández-Mateos P, Cardinali DP, Esquifino AI (2012) Cadmium as an endocrine disruptor: correlation with anterior pituitary redox and circadian clock mechanisms and prevention by melatonin. Free radical biol med. 53(12): 2287-97.
- Poliandri AH, Esquifino AI, Cano P, Jiménez V, Lafuente A, et al., (2006) In vivo protective effect of melatonin on cadmium‐induced changes in redox balance and gene expression in rat hypothalamus and anterior pituitary. J pineal res. 41(3): 238-46.
- Poliandri AH, Machiavelli LI, Quinteros AF, Cabilla JP, Duvilanski BH (2006) Nitric oxide protects the mitochondria of anterior pituitary cells and prevents cadmium-induced cell death by reducing oxidative stress. Free Radic Biol Med. 40(4): 679-88.
- Zheng J-L, Yuan S-S, Wu C-W, Lv Z-M (2016) Acute exposure to waterborne cadmium induced oxidative stress and immunotoxicity in the brain, ovary and liver of zebrafish (Danio rerio). Aquat Toxicol. 180: 36-44.
- Djuric A, Begic A, Gobeljic B, Ninkovic M, Stanojevic I, et al., (2015) Oxidative stress, bioelements and androgen status in testes of rats subacutely exposed to cadmium. Food Chem Toxicol. 86: 25-33.
- Wang J, Zhu H, Liu X, Liu Z (2014) N-acetylcysteine protects against cadmium- induced oxidative stress in rat hepatocytes. J vet sci. 15(4): 485-93.
- Ekpenyong EE, Anderson EL, Towobola AA, Akingbade AM, Kunlere OE (2015) The impact of mimosa pudica on the histoarchitecture of hypothalamic - pituitary -testicular axis in cadmium treated rats. world J pharm pharmaceut sci. 4(10): 163-79.
- Favorito R, Grimaldi MC, Coppola M, Ferrandino I (2010) Effects of acute cadmium exposure on the pituitary gland of Podarcis sicula. Open Zool J. 3(2): 30-6.
- Poliandri AH, Velardez MO, Cabilla JP, Bodo CC, Machiavelli LI, et al., (2004) Nitric oxide protects anterior pituitary cells from cadmium-induced apoptosis. Free Radic Biol Med. 37(9): 1463-71.
- Poliandri AH, Cabilla JP, Velardez MO, Bodo CC, Duvilanski BH (2003) Cadmium induces apoptosis in anterior pituitary cells that can be reversed by treatment with antioxidants. Toxicol appl pharmacol. 190(1): 17-24.
- Erboga M, Kanter M, Aktas C, Donmez YB, Erboga ZF, et al., (2016) Anti-apoptotic and anti-oxidant effects of caffeic acid phenethyl ester on cadmium-induced testicular toxicity in rats. Biol trace element res. 171(1): 176-84.
- Chang K-C, Hsu C-C, Liu S-H, Su C-C, Yen C-C, et al., (2013) Cadmium induces apoptosis in pancreatic β-cells through a mitochondria-dependent pathway: the role of oxidative stress-mediated c-Jun N-terminal kinase activation. PloS one. 8(2): e54374.
- Skipper A, Sims JN, Yedjou CG, Tchounwou PB (2016) Cadmium Chloride Induces DNA Damage and Apoptosis of Human Liver Carcinoma Cells via Oxidative Stress. Int j envir res public health. 13(1): 88.
- Deorukhkar A, Ahuja N, Mercado AL, Diagaradjane P, Raju U, et al., (2015) Zerumbone increases oxidative stress in a thiol‐dependent ROS‐independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer med. 4(2): 278-92.
- Yan Y, Bian JC, Liu XZ, Zhang Y, Ya S, Liu ZP (2012) Oxidative stress and apoptotic changes of rat cerebral cortical neurons exposed to cadmium in vitro. Biomed Environ Sci. 25(2): 172-81.
- Tokumoto M, Fujiwara Y, Shimada A, Hasegawa T, Seko Y, et al., (2011) Cadmium toxicity is caused by accumulation of p53 through the downregulation of Ube2d family genes in vitro and in vivo. J toxicol scie. 36(2): 191-200.
- Lee J-Y, Tokumoto M, Hattori Y, Fujiwara Y, Shimada A, et al., (2016) Different Regulation of p53 Expression by Cadmium Exposure in Kidney, Liver, Intestine, Vasculature, and Brain Astrocytes. Toxicol Res. 32(1): 73-80.
- Teslariu O, Agoroaei L, Mititelu-Tartau L, Zamfir C, Teslariu E, et al., (2016) Experimental researches regarding the montelukast influence in gentamicin induced acute nephrotoxicity. Farmacia. 64(2): 252-6.
- Kim DW, Kim YH, Yousaf AM, Kim DS, Kwon TK, et al., (2016) Novel montelukast sodium-loaded stable oral suspension bioequivalent to the commercial granules in rats. Arch pharm res. 39(4): 539-46.
- Erşahin M, Çevik Ö, Akakın D, Şener A, Özbay L, et al., (2012) Montelukast inhibits caspase-3 activity and ameliorates oxidative damage in the spinal cord and urinary bladder of rats with spinal cord injury. Prostaglandins other lipid mediat. 99(3): 131-9.
- Saad M, Abdelsalam R, Kenawy S, Attia A (2015) Montelukast, a cysteinyl leukotriene receptor-1 antagonist protects against hippocampal injury induced by transient global cerebral ischemia and reperfusion in rats. Neurochem res. 40(1): 139-50.
- Al-Amran FG, Hadi NR, Hashim AM (2013) Cysteinyl leukotriene receptor antagonist montelukast ameliorates acute lung injury following haemorrhagic shock in rats. Eur J Cardio-Thoracic Surg. 43(2): 421-7.
- Cuciureanu M, Căruntu I-D, Păduraru O, Stoica B, Jerca L, et al., (2009) The protective effect of montelukast sodium on carbon tetrachloride induced hepatopathy in rat. Prostaglandins other lipid mediat. 88(3): 82-8.
- Abdel-Raheem IT, Khedr NF (2014) Renoprotective effects of montelukast, a cysteinyl leukotriene receptor antagonist, against methotrexateinduced kidney damage in rats. Naunyn-Schmiedeberg's arch pharmacol. 387(4): 341-53.
- İçer M, Zengin Y, Gunduz E, Dursun R, Durgun HM, et al., (2016) Is montelukast as effective as N-acetylcysteine in hepatic injury due to acetaminophen intoxication in rats? Exp Toxicol Pathol. 68(1): 55-9.
- Dengiz GO, Odabasoglu F, Halici Z, Cadirci E, Suleyman H (2007) Gastroprotective and antioxidant effects of montelukast on indomethacin-induced gastric ulcer in rats. J pharmacol sci. 105(1): 94-102.
- Ahmed AA (2009) Protective effect of montelukast on paraquat-induced lung toxicity in rats. Biosci trends. 3(2): 63-72.
- Dilek F, Ozkaya E, Kocyigit A, Yazici M, Guler EM, et al., (2016) Plasma total thiol pool in children with asthma: Modulation during montelukast monotherapy. Int j immunopathol pharmacol. 29(1): 84-9.
- Khodir A, Ghoneim H, Rahim M, Suddek G (2015) Montelukast attenuates lipopolysaccharide-induced cardiac injury in rats. Hum exp toxicol. 2015: 0960327115591372.
- Moon YJ, Lee JY, Oh MS, Pak YK, Park KS, et al., (2012) Inhibition of inflammation and oxidative stress by Angelica dahuricae radix extract decreases apoptotic cell death and improves functional recovery after spinal cord injury. J neurosci res. 90(1): 243-56.
- Macchi B, Di Paola R, Marino-Merlo F, Rosa Felice M, Cuzzocrea S, et al., (2015) Inflammatory and cell death pathways in brain and peripheral blood in Parkinson’s disease. CNS Neurol Disord Drug Targets. 14(3): 313-24.