Cleaning Effectiveness of EndoActivator Irrigation After Single-File and Multi-File Instrumentation Systems
Salman MI*
Assistant Professor, Department of Conservative Dentistry and Endodontics, Faculty of Dentistry, Mansoura University, Mansoura, Egypt.
*Corresponding Author
Mohamed Ibrahim Salman,
Assistant Professor. Endodontics, Mansoura University, Egypt.
Tel: 00966535387979
E-mail: dr.mohamed.ibrahim@qudent.org
Article Type: Research Article
Received: October 30, 2014; Accepted: January 09, 2015; Published: January 14, 2015.
Citation:Salman IM (2015) Cleaning Effectiveness of EndoActivator Irrigation after Single-File and Multi-File Instrumentation Systems. Int J Dentistry Oral Sci. 2(1), 35-38.doi: dx.doi.org/10.19070/2377-8075-150008
Copyright: Salman IM© 2015. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: To compare cleaning effectiveness of two reciprocating single-file systems with ProTaper rotary instruments after one minute EndoActivator irrigation.
Methods: Thirty three extracted human maxillary and mandibular single rooted human teeth were divided into three
groups. Canals were prepared to the same apical size (25/.08) using Reciproc (Group I), WaveOne (Group II) or F2 ProTaper (Group III). Then all canals received additional one minute activation by EndoActivator size 25/.04. Roots were split and examined with SEM, the presence of debris and smear layer on coronal, middle and apical thirds was evaluated. Data were analyzed with the Kruskal-Wallis and Man-Whitney tests.
Results: One minute EndoActivator irrigation after ProTaper instrumentation achieved significantly better results (P < 0.05) than after instrumentation with either WaveOne or Reciproc. No significant differences were obtained comparing instrumentation with Reciproc or WaveOne (P > 0.05).
Conclusion: EndoActivator irrigation after a multi-file rotary instrumentation resulted in less debris and smear layer than
single-file rotary systems.
2.Introduction
3.Materials and Methods
3.1 Group I
3.2 Group II
3.3 Group III
3.4 Root sectioning and scanning electron microscopy imaging
3.5 Scanning electron microscopy evaluation and statistical analysis
3.6 Statistical analysis
5.Results
6.Discussion
7.References
Introduction
The complexity of the canal anatomy makes it very difficult to efficiently clean all ramifications of the root canal system. Different devices and techniques have been proposed to improve canal cleanliness. Instrumentation of the canal is an essential part of chemomechanical canal preparation. It facilitates removal of pulp tissue and debris and enhances bacterial elimination through apical delivery of irrigant [1]. Many rotary instrumentation systems are available to achieve these goals. Despite variations in file design, fabrication, and technique, significant portions of the canal are untouched, and some debris remains [2-4].
The recently introduced nickel-titanium (NiTi) files Reciproc (VDW, Munich, Germany) and WaveOne (Dentsply Maillefer,
Ballaigues, Switzerland) are claimed to be able to completely prepare and clean root canals with only one instrument. These files are made of a special M-Wire NiTi-alloy that is created by an innovative thermal-treatment process. These files are used in a reciprocal motion that requires special automated devices. Reciproc files are available in different sizes 25, taper 08; 40, taper 06; 50, taper 05 and WaveOne are available in the sizes 21, taper 06; 25, taper 08; and 40, taper 08 [5].
The manufacturer does not strictly recommend creating a glide path when using either Reciproc or WaveOne instruments. Using such instruments maintained the original canal curvature compared to multi-file systems and improved debris and smear layer removal [5]. The main concern of using single-files is the less volume of irrigants used compared to multi-file systems. Alves et al. 2012 [6], demonstrated comparable elimination of bacteria of single-file versus multi-file systems provided the width of apical preparation, volume of irrigants and duration of irrigation are kept similar.
The irrigation of the root canal is an essential procedure in the endodontic treatment for the removal of the smear layer. Currently, the activation of the irrigant is considered because it results in cleaner areas when compared with conventional irrigation [7,8], increases tissue dissolution [9], and significantly reduces the number of bacteria present inside the root canal system [10,11].
The EndoActivator (EA) (Dentsply/Tulsa Dental Specialties, Tulsa, OK) is a cordless, battery-powered handpiece with a sonic motor. It was introduced to improve the irrigation phase. This statement is based on the proposed ability of EA to produce vigorous intracanal fluid agitation. It has been shown to better irrigate the simulated lateral canals at 4.5 and 2 mm from working length as compared with traditional needle irrigation alone [12], and it reportedly removed the smear layer used with demineralizing agents like EDTA and dislodged clumps of simulated biofilm within the curved canals of molar teeth [13].
The purpose of this study was to compare the effectiveness of one minute agitation of 2.5% NaOCl by EA on debris and smear layer removal after root canal preparation with single file systems (WaveOne and Reciproc) versus a multi file sequence (Protaper Universal).
Materials and Methods
Thirty three extracted human maxillary and mandibular single rooted human teeth were selected for this study. K-type files (size 10 or 15) were used to determine working lengths (WL) by subtracting 1 mm from the lengths of the files when they extruded just beyond the apical foramen. Teeth were divided into three groups according to the instrumentation technique used:
A R25 Reciproc file (25/.08) was used in a reciprocating, slow in-and-out pecking motion according to the manufacturer’s instructions. The flutes of the instrument were cleaned after three in and-out-movements (pecks).
Canals were shaped with Primary WaveOne reciprocating files (25/.08), using a reciprocating, slow, in-and-out pecking motion until the full WL was reached.
In either group I or group II, the flutes of the instrument were cleaned after three pecks; at each cleaning, canals were irrigated with 2 ml of 2.5% NaOCl. After complete shaping, all canals received 2 ml of 2.5% NaOCl irrigation. The Single-file dedicated reciprocating motor was used with the manufacturer’s configuration. No glide path was created prior to instrumentation with either the R25 file or WaveOne primary.
ProTaper instruments were used in a modified crown-down manner according to the manufacturer’s instructions using a gentle in-and-out motion till reaching F2 instrument (25/.08). 2 ml of 2.5% NaOCl was used to irrigate canals during and after instrumentation with each file.
The following irrigation protocols was used after shaping by each system:
The EndoActivator System was used to activate 2.5% NaOCl for 1 min. A size 25 0.04 taper polymer tip was used for all three test groups. The EA tip was activated at 10,000 cycles per minute using pumping action in short, 2–3 mm vertical strokes, as recommended by the manufacturer.
After instrumentation and irrigation, root canals were dried with paper points and root orifices were closed with cotton pellets and Cavit (3M ESPE, Seefeld, Germany) to block the entry of debris during sectioning. The roots were split longitudinally in a buccolingual direction and dried in a sequence of increasing concentrations of Ethanol. The two halves (mesial and distal) of each root were mounted on scanning electron microscopy (SEM) carriers with Leit-C (Neubauer, Telgte, Germany). The surfaces were coated with 50 μm of gold layer in an agar sputter coater (Baltec, Balzers, Liechtenstein). Coded specimens were then investigated by SEM (Amray, Bedford, MA, USA). Representative SEM images were obtained for the apical, middle, and coronal sections of each root canal at four different magnifications. Representative regions were selected for each third by screening at low magnification; the selected areas were then analyzed at higher magnification.
SEM images of the root canals were scored for the presence or absence of debris (200× magnification) and smear layer (1000× magnification) at the apical, middle, and coronal regions of each canal, according to a scale developed by Hülsmann et al. [14] as follows:
Debris (dentine chips, pulp remnants and particles loosely attached to the canal wall):
• Score 1: clean canal wall, only very few debris particles.
• Score 2: few small conglomerations.
• Score 3: many conglomerations; less debris than 50 % of the canal wall covered.
• Score 4: more than 50% of the canal wall covered.
• Score 5: complete or nearly complete covering of the canal wall by debris.
Smear layer (a surface film of debris retained on dentine and other surfaces after instrumentation with either rotary instruments or endodontic files; the film consists of dentine particles, remnants of necrotic pulp tissue, bacterial components and retained irrigant).
1. Score 1: no smear layer, orifice of dentinal tubules patent.
2. Score 2: small amount of smear layer, some open dentinal tubules.
3. Score 3: homogenous smear layer along almost the entire canal wall, only very few open dentinal tubules.
4. Score 4: the entire root canal wall covered with a homogenous smear layer, no open dentinal tubules.
5. Score 5: a thick, homogenous smear layer covering the entire root canal wall.
Two trained evaluators independently rated each masked root canal. Conflicting results between these two evaluators were evaluated separately by another trained evaluator to obtain a final evaluation.
Data were analyzed by using the Kruskal–Wallis test and the Mann–Whitney rank-sum test for pairwise comparisons. Differences between groups were considered statistically significant when P ≤ 0.05. All statistical analyses were performed with SPSS 17.0 software (SPSS Inc., Chicago, IL, USA).
Results
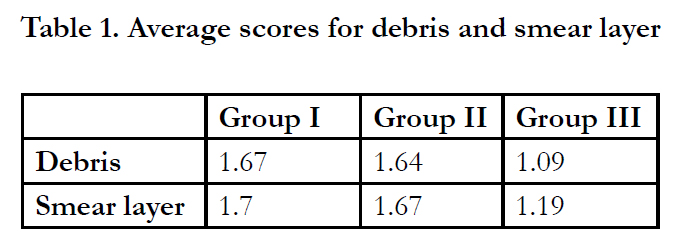
The percentage of scores for debris and smear layer are shown in Figures 1 and 2. When comparing the amount of debris removal by all 3 groups, Group III significantly removed more debris compared to Group I and II (P < 0.05). There was no statistically significant difference between Group I and II (P = 0.977).
In terms of smear layer, no significant difference was found between Group I and II (P = 0.838) while Group III significantly removed more smear layer than groups I and II (P < 0.01). The average scores of debris and smear layer are listed in Table1.
Discussion
Supplementing the effect of irrigants using sonic or ultrasonic devices have been proposed to improve root canal cleanliness and disinfection. The results of this study showed that 1 min agitation of NaOCl improved root canal cleanliness after a full-sequence rotary system (ProTaper) compared to single-file reciprocating systems (Reciproc or WaveOne) (P < 0.05). These results are in agreement with previous studies that showed better debris and smear layer removal after irrigant agitation with EA [13,15,16]. In contrast to our findings, Jensen et al. [17] found no significant difference in cleaning efficiency between sonically and ultrasonically activated files. This was confirmed by Uroz-Torres et al. [18] who found that EA System did not enhance the removal of smear layer as compared with conventional Max-I-Probe irrigation with NaOCl and EDTA.
These differences in results is attributed to the use of passive mode of agitation in the study of Jensin et al. [17], while in this study, The better effect of EA agitated irrigation may be explained by The vertical-stroke pumping motion used as part of the protocol which promotes the formation of more microbubbles alongside the EA file that gradually increases in diameter until they collapse provoking very effective small implosions, which produce an irregular agitation of the irrigant [13,19].
The efficacy of EA was improved using larger tip sizes and high power setting [20], these findings may explain the better results in this study, where a polymer tip size 25, 0.04 taper was used to agitate the hypochlorite solution while in the study of Urezz Tores [18], size 15, 0.02 tip was used.
The data show that no significant differences were obtained comparing instrumentation with Reciproc or WaveOne (P > 0.05). This may be explained by the minimal time of instrumentation by both systems [5] that may not have permitted enough contact time for the NaOCl to aid in debris or smear layer removal.
Within the limitations of our study, the EndoActivator System did not enhance the removal of debris or smear layer after single-file instrumentation pointing to the importance of adjusting the volume and contact time of the irrigants when using these systems.
References
- Hülsmann M, Peters OA, Dummer PM (2005)Mechanical preparation of root canals: shaping goals, techniques and means. Endodontic topics 10(1):30-76.
- Paque F, Ganahl D, Peters OA (2009) Effects of root canal preparation on apical geometry assessed by micro-computed tomography. J Endod 35(7):1056-1059.
- Peters OA, Schonenberger K, Laib A (2001) Effects of four Ni-Ti preparation techniques on root canal geometry assessed by micro computed tomography. Int Endod J 34(3):221-230.
- Wu MK, Wesselink PR (2001) A primary observation on the preparation and obturation of oval canals. Int Endod J 34(2):137-141.
- Bürklein S, Hinschitza K, Dammaschke T, Schafer E (2012) Shaping ability and cleaning effectiveness of two single-file systems in severely curved root canals of extracted teeth: Reciproc and WaveOne versus Mtwo and ProTaper. Int Endod J 45(5):449-461.
- Alves FR, Rocas IN, Almeida BM, Neves MA, Zoffoli J et al (2012) Quantitative molecular and culture analyses of bacterial elimination in ovalshaped root canals by a single-file instrumentation technique. Int Endod J 45(9):871-877.
- van der Sluis LW, Gambarini G, Wu MK, Wesselink PR (2006) The influence of volume, type of irrigant and flushing method on removing artificially placed dentine debris from the apical root canal during passive ultrasonic irrigation. Int Endod J 39(6):472-476.
- Salman MI, Baumann MA, Hellmich M, Roggendorf MJ, Termaat S (2010) SEM evaluation of root canal debridement with Sonicare CanalBrush irrigation. Int Endod J 43(5):363-369.
- Gutarts R, Nusstein J, Reader A, Beck M (2005) In vivo debridement efficacyof ultrasonic irrigation following hand-rotary instrumentation in human mandibular molars. J Endod 31(3):166-170.
- Carver K, Nusstein J, Reader A, Beck M (2007) In vivo antibacterial efficacy of ultrasound after hand and rotary instrumentation in human mandibular molars. J Endod 33(9):1038-1043.
- Pasqualini D, Cuffini AM, Scotti N, Mandras N, Scalas D et al (2010) Comparative evaluation of the antimicrobial efficacy of a 5% sodium hypochlorite subsonic-activated solution. J Endod 36(8):1358-1360.
- de Gregorio C, Estevez R, Cisneros R, Heilborn C, Cohenca N (2009) Effect of EDTA, sonic, and ultrasonic activation on the penetration of sodium hypochlorite into simulated lateral canals: an in vitro study. J Endod 35(6):891-895.
- Caron G, Nham K, Bronnec F, Machtou P (2010) Effectiveness of different final irrigant activation protocols on smear layer removal in curved canals. J Endod 36(8):1361-1366.
- Hülsmann M, Rummelin C, Schafers F (1997) Root canal cleanliness after preparation with different endodontic handpieces and hand instruments: a comparative SEM investigation. J Endod 23(5):301-306.
- Blank-Goncalves LM, Nabeshima CK, Martins GH, Machado ME (2011) Qualitative analysis of the removal of the smear layer in the apical third of curved roots: conventional irrigation versus activation systems. J Endod 37(9):1268-1271.
- Kanter V, Weldon E, Nair U, Varella C, Kanter K et al (2011) A quantitative and qualitative analysis of ultrasonic versus sonic endodontic systems on canal cleanliness and obturation. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 112(6):809-813.
- Jensen SA,Walker TL,Hutter JW, Nicoll BK (1999) Comparison of the cleaning efficacy of passive sonic activation and passive ultrasonic activation after hand instrumentation in molar root canals. J Endod 25(11):735-738.
- Uroz-Torres D,Gonzalez-RodriguezMP, Ferrer-Luque CM (2010) Effectiveness of the EndoActivator System in removing the smear layer after root canal instrumentation. J Endod 36(2):308-311.
- Shen Y, Stojicic S, Qian W, Olsen I, Haapasalo M (2010) The synergistic antimicrobial effect by mechanical agitation and two chlorhexidine preparations on biofilm bacteria. J Endod 36(1):100-104.
- Huang TY, Gulabivala K, Ng YL (2008) A bio-molecular film ex-vivo model to evaluate the influence of canal dimensions and irrigation variables on the efficacy of irrigation. Int Endod J 41(1):60-71.