Effect of Nano Filler on Microhardness, Diametral Tensile Strength and Compressive Strength of Nano-Filled Glass Ionomer
Toras FM1, Hamouda IM2,3*
1 Student, Faculty of Dentistry, Umm Al-Qura University, Makkah, Saudi Arabia.
2 Professor of Dental Biomaterials, Faculty of Dentistry, Mansoura University, Mansoura, Egypt.
3 Head of Conservative Dentistry, Faculty of Dentistry, Umm Al-Qura University, Makkah, Saudi Arabia.
*Corresponding Author
Ibrahim Mohamed Hamouda,
Head of Conservative Dentistry,
Faculty of Dentistry,
Umm Al-Qura University, Makkah, Saudi Arabia.
Tel: +966542812148
Fax: 0020502260173
E-mail: imh100@hotmail.com
Received: January 15, 2017; Accepted: February 07, 2017; Published: February 08, 2017
Citation: Toras FM, Hamouda IM (2017) Effect of Nano Filler on Microhardness, Diametral Tensile Strength and Compressive Strength of Nano-Filled Glass Ionomer. Int J Dentistry Oral Sci. 4(2), 413-417. doi: dx.doi.org/10.19070/2377-8075-1700082
Copyright: Hamouda IM© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: This study was designed to evaluate the microhardness, diametral tensile strength and compressive strength of a nano-filled glass ionomer restorative material in comparison with a resin modified and conventional glass ionomers.
Materials and Methods: A total of 45 specimens were prepared from three types of glass ionomer restorative materials. Specimens were cured with a light curing unit according to the manufacturer’s instructions. The specimens were prepared using Teflon split molds with dimensions of 6 mm height and 4 mm diameter. Incremental curing in 3 layers, 2mm each were used. After curing, all specimens were immersed in distilled water for 24 hours. Microhardness test was done using a Vickers microhardness Tester with 25 gf load for 5 sec. Diametral tensile
strength and compressive strength were measured using Intestron testing machine at cross-head speed of 50 gm/sec until fracture.
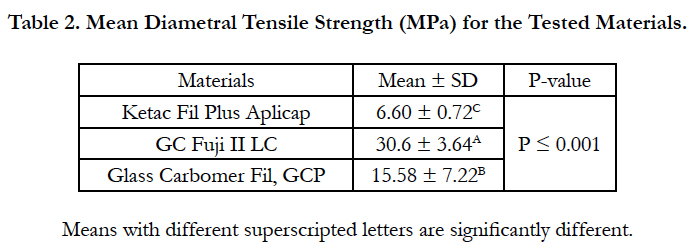
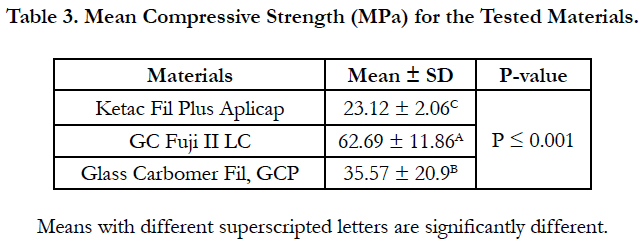
Results: There was a significant difference among the tested materials in all testing parameters. Ketac fil Aplicap exhibited the highest hardness value while Glass carbomer fil GCP has the lowest value while GC Fuji II LC showed intermediate micro-hardness value. GC Fuji II LC exhibited the highest diametral tensile and compressive strength values while Ketac Fil Plus Aplicap has the lowest values. Glass Carbomer Fil, GCP showed intermediate diametral tensile and compressive strength values.
Conclusions: The nano-filled glass ionomer (Glass Carbomer Fil, GCP) exhibited the lowest hardness value, intermediate diametral tensile and compressive strength values.
2.Introduction
3.Materials and Methods
3.1 Microhardness Testing
3.2 Diametral Tensile Strength Testing
3.3 Compressive Strength Testing
3.4 Statistical Analysis
4.Results
4.1 Microhardness
4.2 Diametral Tensile Strength
4.3 Compressive Strength
5.Discussion
6.Conclusions
7.References
Keywords
Glass Ionomer; Restorative Material; Nanofilled; Resin-Modified; Microhardness; Tensile Strength and Compressive Strength.
Introduction
In seventies, amalgam was used commonly as direct restoration, while ceramic and gold were used as indirect restoration. Recent studies have limit the use of amalgam for many reasons like the potentiality of toxicity and aesthetic matter. Nowadays, composite and glass ionomer are the main tooth-colored restoration used for direct restoration providing less allergic reaction or toxicity and great appearance [1, 2].
Many years ago, there was an obvious development and great modifications in dental materials generally and in glass ionomer specifically. Glass ionomer restorative materials were introduced in the seventies, modifications and improvements were done. Clinical advantages of glass-ionomers include fluoride release, ability to adhere to moist enamel and dentin and radiopacity. Glass ionomer is the only material that self-adhere to tooth. The different types with different characteristics reflect this improvement [1, 3]. The different types depend mainly on the different chemical formulation as follow: conventional glass ionomer, resin-modified glass ionomer and nano-filled glass ionomer [1, 4].
Conventional glass ionomer or chemically cured glass ionomer was firstly introduced to the market. It has great appearance as it was a tooth colored materials. The main disadvantage for this type is poor physico-mechanical properties. Resin modified glass ionomer is a dual cured: self setting and light cured. It comes after conventional glass ionomer to overcome the physico-mechanical obstacle by adding resin [1, 3]. It have many advantages in comparison to conventional one and that is better physico-mechanical properties, less micro-leakage and better adhesion to enamel and dentin [1, 5]. More recently, with the advent of ART (atraumatic restorative technique), there was the need to improve the physical properties of these materials, leading then to high viscosity GICs, with chemical activation. These materials have a greater number of particles with smaller sizes [6].
Recent type of glass ionomer is Nano-Filled glass ionomer. The use of nanoparticles, generally defined as particles smaller than 100 nm. It plays a role in improvement of physical properties [1, 7]. This study was designed to evaluate the microhardness, diametral tensile strength and compressive strength of a nano-filled glass ionomer restorative material in comparison with a resin modified and conventional glass ionomer restorative materials.
Materials and Methods
The materials used in this study were a conventional glass ionomer (Ketac Fil Plus Aplicap, 3M ESPE, Germany), resin modified glass ionomer (GC Fuji II LC, GC Corporation, Japan) and nanofilled glass ionomer (Glass Carbomer Fil, GCP Dental, Netherlands).
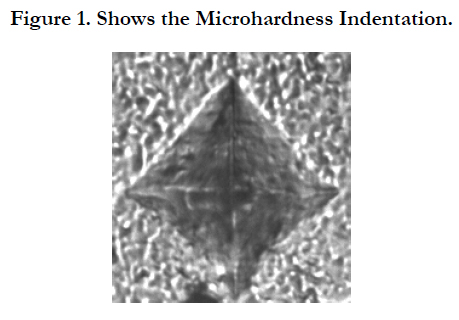
15 cylindrical specimens were prepared with dimension of 6 mm height and 4 mm diameter using Teflon molds, 5 specimens from each material. The materials were prepared according to the manufacturer’s instructions. Celluloid strip was placed on top of the filled mold. Slight pressure was applied on the filled mold using a microscopic glass slide to extrude the excess material. The glass slide was removed and the material was cured using a light curing unit (3 M Espe, Elipar, Light-cure, Germany). Incremental curing in 3 layers, 2mm each was followed. After curing, all specimens were immersed in distilled water for 24 hours. Microhardness measurements were done using a Vickers microhardness Tester (Wilson hardness, Buehler, USA). A 25 gf load was applied for 5 sec indentation time via the Vickers diamond pyramid producing surface indentation on the specimen (Figure 1). Five readings were taken for each specimen. Total mean Vickers microhardness (VHN) was computed.
15 specimens with dimensions of 6 mm diameter and 4 mm height were prepared using Teflon molds (Figure 2). Incremental curing was done, after curing, all specimens were immersed in distilled water for 24 hours. Specimens were placed with the flat ends perpendicular to the platens of the testing machine. The load was applied to the diameter of the specimen using a universal testing machine (INSTRON 5944 Tester, U.S.A.) with cross-head speed 50 gm/min, until fracture.
The maximum load was applied to fracture the specimen and recorded in Newton. Diametral tensile strength (DTS) was calculated in MPa using the following formula:
DTS = 2F/π dt
Where F is the maximum applied load (N), d is the diameter of the specimen (mm) and t is the thickness of the specimen (mm).
15 Cylindrical specimens were prepared with dimension of 6 mm in height and 4 mm in diameter using Teflon split molds according to the manufacturer’s instructions. The composite was applied incrementally in three layers and cured separately. A Celluloid strip was placed on top of the filled mold, then a second glass plate was placed above the Celluloid strip. Slight pressure was applied and the excess material was removed. The specimens were separated from their molds and kept in distilled water for 24 hours. Each specimen was placed with the flat surface between the platens of the testing machine, so that, the load was applied on the long axis of the specimens. The maximum load at fracture of the specimen was recorded in (N). The compressive strength was calculated using this formula:
Compressive strength = 4P/π d2 (MPa).
Where P is the maximum applied load (N) and d is the diameter of the specimen (mm).
Data were collected, tabulated and subjected to statistical analysis using SPSS program version 22.0. The comparison within groups was carried out using One-way ANOVA test and between groups using Least Significance Difference (LSD) test. The level of significance was considered at P value ≤ 0.05.
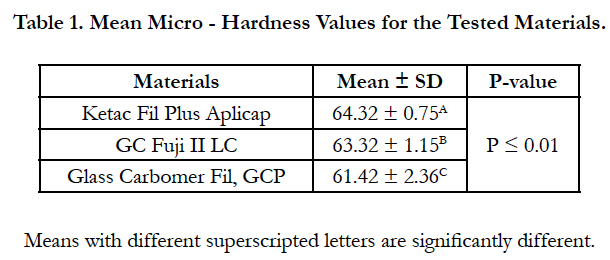
The results of the micro-hardness test are presented in Table 1. One-way ANOVA showed significant difference among the tested materials (P < 0.01). Ketac fil Aplicap exhibited the highest hardness value while Glass carbomer fil GCP has the lowest value. GC Fuji II LC showed intermediate micro-hardness value. There was no significant difference between Ketac fil Aplicap and Fuji II LC (P ≥ 0.05). There was a significant difference between Fuji II LC and Glass Carbomer fil GCP (P ≤ 0.05).There was a significant difference between ketac fil Aplicap and Glass Carbomer fil GCP (P ≤ 0.05).
The results of diametral tensile strength test are presented in Table 2. One-way ANOVA showed significant difference among the tested materials (P ≤ 0.001). GC Fuji II LC exhibited the highest diametral tensile strength values while Ketac Fil Plus Aplicap has the lowest values. Glass Carbomer Fil, GCP showed intermediate diametral tensile strength values. There were highly significant differences between the tested materials (P ≤ 0.001).
The results of the compressive strength test are presented in Table 3. One-way ANOVA test showed a significant difference among the tested materials (P ≤ 0.001). GC Fuji II LC exhibited the highest compressive strength values while ketac fil Aplicap has the lowest values. Glass Carbomer fil GCP showed intermediate compressive strength values. There were highly significant differences between the tested materials (p ≤ 0.001).
Discussion
The analysis of the compressive strength, diametral tensile strength and microhardness are important for verification and comparison of mechanical properties of different dental materials and may reflect what material is best suitable to perform clinical functions and resisting to the masticatory forces [3, 8]. The present study included the analysis of three materials, aiming to determine which of these materials is the most suitable for clinical application in restoration of teeth. Major disadvantages of glass ionomers in use as a restorative materials include its weak mechanical properties, such as low wear resistance and low fracture toughness [1, 7]. This makes it unsuitable for use in high-stress areas such as Class I and II restorations [1].
Microhardness test is a parameter frequently used to evaluate the material surfaces resistance to plastic deformation by penetration [3]. In restorative dentistry, the resin-modified glass ionomer materials, the fillers are considered the strongest phase. Their primary purpose is to strengthen the conventional glass ionomer and to reduce the amount of weak matrix material, resulting in increased hardness, strength, and decreased wear. Previous studies show that increasing the filler size and content improves the mechanical properties of the material. Also, the nano-filled materisls has higher significant value in comparing with conventional glass ionomer [8]. Regarding the hardness, the present study showed that there was no significant difference between nano-filled glass ionomer and conventional glass ionomer. The highest hardness was noted with the resin-modified glass ionomer.
Compressive tests are used in dentistry for laboratory simulation of the stress that may result from forces applied clinically to a restorative, base/liner or core build material. Most mastication forces are compressive in nature, but exact critical value is unknown [7]. Therefore, it is important to investigate whether compressive force contributes to fracture failure during mastication process. Although the compression specimen has a convenient cylindrical geometry, perfection of the ends (which is essential to produce uniform contact between the specimen and the testing device) is difficult to achieve [1, 7]. The present study showed compressive strengths of different conventional glass ionomer such as Ketac fil and Fuji II LC (GC) were higher than that of glass ionomer studied in other studies, the difference in values can be explained by the size of specimens [2].
A previous study indicated that the compressive strength of the conventional glass ionomer could be increased by addition of nano-filler such as nano-sized TiO2 NPs [9]. The present study used Carbomer glass fil which filled with nano hydroxyapatite and fluorapatite showed higher compressive strength than that of the conventional glass ionmer. The compressive strength values depend on the types of nano-filled particles [10].
Glass Carbomer fil have shown higher diametral tensile strength than conventional glass ionomers. However, Resin-modified glass has presented better scores than both conventional and carbomer materials. Due to the addition of resin, these materials have the normal glass ionomer cement acid-base reaction and a free-radical or photochemical polymerization process [11]. In this system, there is lack of water because it was replaced by a water/HEMA mixture. For this reason, the polymerization of HEMA is the initial set and the acid-base reaction proceeds more slowly. Consequently, there is longer working time, rapid set and early water contamination resistance [12]. The addition of resin improve the mechanical properties but does not negatively affect the other properties of conventional glass ionomers. Fluoride release, for example, is equivalent or higher than conventional materials [13].
The microhardness of the resin modified GI in the present study was below the conventional glass ionomer with no significant difference between them. This result is in agreement with results from a previous study [14]. On the other hand, some author reported that resin modified glass ionomer gives highest result in microhardness test [15]. Their studies showed that nano-filled materials have a significant higher hardness value in comparing with conventional glass ionomer. The present study showed that there was no significant differenence between nano-filled glass ionomer and conventional glass ionomer. This difference may be attributed to the differences in the particles type, they used titanium dioxide (TiO2) nanoparticles instead of nano hydroxyapatite and fluorapatite [8, 16].
In the present research the compressive strength of resin modified GI (Fuji II LC) was higher than that of the conventional glass ionomer. Some author suggests that compressive strength of resin modified glass ionomer gives low reading when adding bioactive glass (BAG) particles [17]. However, other studies indicated that the addition of nano hydroxyapatite and fluorapatite improved the compressive strength when compared to the conventional glass ionomer [16]. Other study indicated that addition of nano-sized TiO2 particles increased the compressive strength [9].
Rupture under low tension characterizes fragile materials, susceptible to brittleness. In these cases, tensile strength is not indicated to evaluate material reaction, because of the low cohesive condition. An alternative method of tensile strength is calculated by compressive testing [7]. Nano-filled glass ionomer have shown higher diametral tensile strength than conventional glass ionomer while the resin-modified glass ionomer have presented better scores than both conventional and nano-filled materials [18, 19].
Conclusions
within the limitations of this study, the following conclusions were drawn:
1. There was a significant difference among the tested materials in all testing parameters.
2. Ketac fil Aplicap exhibited the highest hardness values while Glass carbomer fil GCP has the lowest values. GC Fuji II LC showed intermediate micro-hardness values.
3. GC Fuji II LC exhibited the highest diametral tensile and compressive strength values while Ketac Fil Plus Aplicap has the lowest values. Glass Carbomer Fil, GCP showed intermediate diametral tensile and compressive strength values.
4. The nano-filled glass ionomer (Glass Carbomer Fil, GCP) exhibited the lowest hardness and intermediate diametral tensile and compressive strength values.
References
- Anusavice KJ, Phillips (1996) Science of Dental Materials. 10th (Edn),W.B. Saunders Co, Philadelphia, 63.
- Lohbauer U (2010) Dental Glass Ionomer Cements as Permanent Filling Materials? -Properties, Limitations and Future Trends: a review. Materials. 3(1): 76-96.
- Bonifacio CC, Kleverlaan CJ, Raggio DP, Werner A, de Carvalho RCR, et al., (2009) Physical-mechanical properties of glass ionomer cements indicated for atraumatic restorative treatment. Aust Dent J. 54(3): 233–237.
- Zoergiebel J, Ilie N (2013) Evaluation of a conventional glass ionomer cement with new zinc formulation: effect of coating, aging and storage agents. Clin Oral Investig. 17(2): 619-626.
- Strassler HE (2011) Glass Ionomers For Direct-Placement Restorations. A Peer-Reviewed Publ, Switzerland.
- Rizzante FAP, Cunali RS, Bombonatti JFS, Correr GM, Gonzaga CC, et al., (2015) Indications and restorative techniques for glass ionomer cement. Literature review article, RSBO. 12(1): 79-87.
- Cattani-Lorente MA, Godin C, Meyer JM (1994) Mechanical behavior of GICs affected by long-term storage in water. Dent Mater. 10(1): 37-44.
- Wang L, Perlatti D’ALpino PH, Lopes LG, Pereira JC (2003) Mechanical properties of dental restorative materials: relative contribution of laboratory tests. J Appl Oral Sci. 11: 162-167.
- Hammouda IM (2009) Reinforcement of conventional glass-ionomer restorative material with short glass fibers. J Mech behav biomed mater. 2(1): 73-81.
- Garcia-Contreras R, Scougall-Vilchis RJ, Contreras-Bulnes R, Sakagami H, Morales-Luckie RA, et al., (2015) Mechanical, antibacterial and bond strength properties of nano-titanium-enriched glass ionomer cement. J Appl Oral Sci. 23(3): 321–328.
- Wilson AD, Kent BE (1971) The glass-ionomer cement: a new translucent dental filling material. J Appl Chem Biotechnol. 21(11): 313.
- McLean JW, Wilson AD (1977) The clinical development of the glass-ionomer cements. 1. Formulations and properties. Aust Dent J. 22(1): 31-36.
- Cho SY, Cheng AC (1999) A review of glass ionomer restorations in the primary dentition. J Can Dent Assoc. 65(9): 491-495.
- Bala O (2012) Evaluation of surface roughness and hardness of different glass ionomer cements. Eur J Dent. 6(1): 79–86.
- AlJamhan AS (2011) In-vitro wear and hardness of new conventional glass ionomer cement coated with nano-filled resin. Master of Science in Dentistry, Indiana University, School of Dentistry.
- Moshaverinia A, Ansari S, Darr JA, Roohpour A, Rehman I, et al., (2008) Effects of incorporation of hydroxyapatite and fluoroapatite nanobioceramics into conventional glass ionomer cements (GIC). Acta Biomater. 4(2):432–440.
- Yli-Urpo H, Narhi T, Vallittu PK, Lassila LV (2005) Compressive strength and surface characterization of glass ionomer cements modified by particles of bioactive glass. Dent Mater. 21(3): 201–209.
- Cefally DFG, Franco EB, Lia-Mondelli RF, Francisconi PAS, Navarro MF (2003) Diametral tensile strength and water sorption of glass-ionomer cements used in atraumatic restorative treatment. J Appl Oral Sci. 11(2): 96-101.
- Molina GF, Cabral RJ, Mazzola I, Lascano LP, Frencken JE (2013) Mechanical performance of encapsulated restorative glass-ionomer cements for use with atraumatic restorative treatment (ART). J Appl Oral Sci. 21(3):243–249.