A Systematic Review of Encapsulation and Control Release Technology in food Application
Melaku Tafese Awulachew*
Ethiopian institute of Agricultural research, Addis Ababa, Ethiopia.
*Corresponding Author
Melaku Tafese Awulachew,
Ethiopian institute of Agricultural research, Addis Ababa, Ethiopia.
E-mail: melakutafese12@gmail.com
Received: August 26, 2021; Accepted: December 22, 2021; Published: December 24, 2021
Citation: Melaku Tafese Awulachew. A Systematic Review of Encapsulation and Control Release Technology in food Application. Int J Dairy Process Res. 2021;4(1):71-78.
Copyright: Melaku Tafese Awulachew© 2021. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
This study is aims to give a brief description ofencapsulation and control release technology in food application with research
reports and verification of well-known common sense in elsewhere, that exist as part of the commonly known and very effective
in food preservation. Besidesthe material give potential information for those who interested for futuredevelopment
perspectives of the sector and also create awareness potentiallyfor readers, traders, Students, factory workers, technologist
and related stakeholder. A process to entrap active agents within a carrier material called Encapsulation and it is a useful
tool to improve delivery of bioactive molecules and living cells into foods. Therefore, encapsulation preserve stability of the
bioactive compounds during processing and storage and to prevent undesirable interactions with food matrix. During encapsulation
process, a large number of substances are used to encapsulate solid or liquid food ingredients. Micro-organisms are
the main agents responsible for food spoilage and food poisoning and therefore food preservation procedures are targeted
towards them. Generally,the selection of encapsulating materials depends on the types, origins, and properties of these food
ingredients.It is being increasingly popular in pharmaceutical, nutraceutical and functional food industries as a highly effective
method that performs various functions; the major being prolonging the shelf-life of the active, masking the undesirable
flavour, colour and taste and controlling the release of bioactive.
2.Introduction
3.Overview Of Encapsulation And Control Release
4.Mechanisms Of Microbial Inactivation
5.Critical Factors Determining Microbial Inactivation
6.Current Emerging Combination Technologies For Food Processing
7.Future Perspective (Development Potentials)
8.Conclusion
9.References
Keywords
Food Preservation; Technology; Encapsulation; Control Release; Future Potential.
Introduction
A process to entrap active agents within a carrier material called
Encapsulation and it is a useful tool to improve delivery of bioactive
molecules and living cells into foods. The encapsulated substance,
except active agent, named as the core, fill, active, internal
or payload phase. The substance that is encapsulating is often
named as the coating, membrane, shell, capsule, carrier material,
external phase, or matrix [1, 2]. In the food industry, encapsulation
process can be applied for a variety of reasons. Encapsulation
is a useful tool to improve delivery of bioactive molecules
(e.g., antioxidants, minerals, vitamins, phytosterols, lutein, fatty
acids, lycopene) and living cells (e.g., probiotics) into foods [1,
3]. In most cases, encapsulation refers to a technology in which
the bioactive components are completely enveloped, covered and
protected by a physical barrier, without any protrusion of the
bioactive components [3]. Produced particles usually have diameters
of a few nm to a few mm [1]. Functional compounds are
used to control flavour, colour, texture or preservation properties.
Bioactive compounds with various potential health benefits are
included, too.
There is a multitude of possible benefits of encapsulated ingredients
in the food industry. Encapsulation aims to preserve stability
of the bioactive compounds during processing and storage and to
prevent undesirable interactions with food matrix. Mainly, bioactive
food compounds are characterized by rapid inactivation. In
addition to the above, encapsulation can be applied for modification
of physical characteristics of the original material in order to
(a) allow easier handling, (b) to help separate the components of
the mixture that would otherwise react with one another, (c) to
provide an adequate concentration and uniform dispersion of an
active agent [4].
These compounds would profit from an encapsulation procedure,
since it slows down the degradation processes (e.g., oxidation or
hydrolysis) or prevents degradation until the product is delivered
at the desired sites [5]. The European Directive (3AQ19a) defines
controlled release as a “modification of the rate or place at which an active substance is released.” Such a modification can be made
using materials with specific barrier properties for manipulating
the release of an active and to provide unique sensory and/or
functional benefits. Addition of small amounts of nutrients to
a food system, for example, may not affect its properties significantly;
however, incorporating high levels of the nutrient either
to meet certain requirements or to treat an ailment will most often
result in unstable and often unpalatable foods. Examples of
such nutrients include fortification with calcium, vitamins, polyunsaturated
fatty acids, and so on, and the associated grittiness,
medicinal and oxidized taste, respectively. Different types of controlled-
release systems have been formulated to overcome these
challenges and to provide a wide range of release requirements.
The two principal modes of controlled release are delayed and
sustained release.
Delayed release is a mechanism whereby the release of an active
substance is delayed from a finite “lag time” up to a point when/
where its release is favored and is no longer hindered. Examples
of this category include encapsulating probiotic bacteria for their
protection from gastric acidity and further release in the lower
intestine, flavor release upon microwave heating of ready-meals
or the release of encapsulated sodium bicarbonate upon baking
of a dough or cake batter.
Sustained release is a mechanism designed to maintain constant
concentration of an active at its target site. Examples of this
release pattern include encapsulating flavors and sweeteners for
chewing gum applications so that their rate of release is reduced
to maintain a desired flavor effect throughout the time of chewing.
A wide range of cores (encapsulants), wall-forming materials
(encapsulating agents), and technologies for controlling the interactions
of ingredients in a given food system and for manufacturing
microcapsules and micro particles of different size, shape, and
morphological properties are commercially viable. Therefore, the
objective of this material is to providebrief overview to the basic
understanding and common process to encapsulate food active
agent and control release system in food processing.
Overview Of Encapsulation And Control Release
Principles and equipment of encapsulation and control release
Wall-Forming Materials: Wall forming materials are a shell material
used for food ingredients encapsulation. Because, a large
number of substances are used to encapsulate liquid or solid food
ingredients; the selection of encapsulating materials depends on
the origin,types, and properties of these food ingredients. Among
various shell materials only some numbers have been certified for
food applications as “overall recognized as safe” materials. In general,
these encapsulating agents are biopolymeric substances such
as lipids, proteins, gums or their derivatives. Materials used in film
coating or matrix formation include several categories:
• Waxes and lipids: Candelilla, beeswax, and carnauba waxes, wax
micro- and wax macroemulsions, modified fats and glycerol distearate,
natural.
• Proteins: soy proteins, gelatins, whey proteins, gluten,zein, and
so on. All these proteins are available both in modified forms and
native.
• Carbohydrates: maltodextrins, starches, chitosan, sucrose, ethyl
cellulose, cellulose acetate, glucose, alginates, chitosan, carrageenan’s,
and so on.
• Food grade polymers: poly vinyl acetate, polypropylene, polybutadiene,
polystyreneand so on.
Carbohydrates: Starch and starch derivatives for instance maltodextrin,
cellulose derivatives for instance carboxymethyl cellulose,
gums for instance gum Arabic, guar gum and chia seed gum and
β-cyclodextrin are the most commonly used carbohydrate-based
wall materials. This is because of their abundant availability, excellent
core protection ability, bland flavour, these wall materials are
used to encapsulate diverse food materials such as oxygen sensitive
and PUFA-rich oil, vitamins, proteins & bioactive peptides,
enzymes and flavour [6-8]. Modified starches are produced by inducing
side chains of lipophilic succinic acid to increase the emulsifying
ability of starch. Moreover, Modified starches are found to
show better protection than native and waxy starch [9] and offer
exciting emulsion stability [10].
Proteins: Superior functional and physicochemical properties
including gel forming ability, emulsifying capacity and film formation
capability make proteinan excellent encapsulating material
which find huge applications in food industries [11, 12]. Gelatin
is the widely used shell matrix used to manufacture highly stable
soft gels of omega-3, vitamin D and fish oil. Milk proteins such
as sodium caseinate and whey protein isolate, and other plant proteins
such as soy proteins, pea proteins have been used as wall
materials for several years. Whey protein has also been reported
as fantastic wall materials for encapsulating sensitive flavours and
PUFA-rich oils. This protein possesses excellent encapsulation efficiency
(up to 89.6%) over other proteins such as soy protein (up
to 75.9%) [13, 14]. The authors found that resultant microcapsules
recovered by spray drying remain stable over 60 days at high
water activity (aw = 0.74 - 0.90) [14]. One of the major limitations
of using protein as encapsulants is their allergenicity to some individuals.
wheat protein (e.g., gluten), Soy proteins, and peanut proteins are
reported to be highly allergenic to a number of individuals. This
not only limits their application but also warrants manufacturer
declaration on the label for their presence in the designed foods.
In addition, proteins are sensitive to structural changes and their
effectiveness as wall materials is greatly dependent such as pH,
ionic strength and temperature of the emulsions or solution [5,
14]. Even Hough, blending these proteins with other materials,
particularly carbohydrate-based biopolymers, such as maltodextrin,
corn syrup solids and lactose has been reported to be an
effective method to minimize environmental effect on their functionality
as encapsulants [15, 16].
Lipids: Since lipids are hydrophobic materials and are insoluble
in water and hence, they are widely used to encapsulate hydrophilic
substances.Many different types of lipids including phospholipids,
glycerides, fatty acidsand waxes have been explored for
their ability to encapsulate food actives [1]. Although lipid-based
encapsulation technology is relatively new and emerging field, it is
becoming highly popular as a means of delivering pharmaceutical,
bioactive food and nutraceutical ingredients. Main types of lipidbased
delivery systems are four: Nano emulsions, nanoliposomes,
solid lipid nanoparticles and nanostructure lipid carriers [17].
Core Materials: Coating substances that are basically film forming
materials can be selected from a wide variety of synthetic
polymers or natural, depending on the characteristics desired in
the final microcapsules the material to be coated. The coating
composition is the main determinant of the functional properties
of the microcapsule and of the method to be used to improve
the performance of a particular ingredient. An effective coating
material should have good rheological properties at high concentration
and ease of manipulation during the process of encapsulation
and also, selected so that it produces a stable emulsion or dispersion
with the active ingredient, and does not react or degrade
the active material during processing and storage. Beside this, it
should meet specified or desired capsule solubility properties and
active material release properties.
Coating materials for encapsulation of food ingredients can be
subdivided into cellulose, gums, lipids, and proteins. Core materials
include flavors, nutraceutical, antimicrobial agents, and
therapeutic actives, vitamins, alkalis, buffers, sweeteners, minerals,
antioxidants, colors, acids, nutrients, enzymes, cross-linking
agents, yeasts, chemical leavening agents, and so on. For instance,
encapsulation by extrusion and spray drying depends primarily
on the carbohydrates used for the encapsulation matrix. Furthermore,
Gums usually used as control crystallization, texturing ingredients,
stabilize emulsions, and inhibit syneresis (the release of
water from fabricated foods), thereby improving coating properties.
Lipids are generally used for encapsulation for water soluble
ingredients. Protein ingredients are also effective in encapsulating
food ingredients. In particular, gelatin is used in coacervation.
Processing technology of encapsulation and control release
Release Triggers: Encapsulation and controlled-release systems
can be designed to respond to one or a combination of triggers
that can activate the release of the entrapped substance and to
meet a desired release target or rate. Triggers can be one or a
combination of the following:
• Temperature: fat/wax matrices
• Moisture: hydrophilic matrices
• pH: enteric coating, emulsion coalescence, and others.
• Enzymes: enteric coating as well as a variety of lipid, starch and
protein matrices.
• Shear: chewing, physical fracture, and grinding
• lower critical solution temperature (LCST) of hydrogels.
The Payload is means to estimate the amount of active (core) entrapped
in a given matrix or wall material (shell) and the percentage
of payload is expressed as Equation: Payload (%) = [core) /
(core + shell)] * 100
Entrapment of Actives in Food Matrices: Encapsulation of
active into an amorphous matrix, generally, involves melting
a crystalline polymer using heat and/or shear to transform the
molecular structure into an amorphous phase. The encapsulant is
then incorporated into the metastable amorphous phase followed
by cooling to solidify the structure and form glass, thus restricting
molecular movements. Carbohydrates are excellent candidates for
encapsulation applications due to the several attributes possessed
by them.
• They form an integral part of many food systems.
• They are cost-effective.
• They occur in a wide range of polymer sizes.
• They have desirable physicochemical properties such as solubility,
melting, phase change and so on.
Sucrose, maltodextrins, native and modified starches, polysaccharides,
and gums have been used in encapsulating flavors, minerals,
vitamins, probiotic bacteria as well as pharmaceutical actives. The
unique helical structure of the amylose molecule, for example,
makes starch a very efficient vehicle for encapsulating molecules
like lipids, flavors, and so on [18]. Some carbohydrates such as
inulin and trehalose can provide additional benefits for encapsulation
applications. Inulin, for example, is a prebiotic ingredient that
can enhance survival of probiotic bacteria while trehalose serves
as a support nutrient for yeasts.
Spray drying and Extrusion are the two main technologies have
been used in large-scale encapsulation applications into amorphous
matrices, though using different mechanisms.
In spray drying, for example, the active is trapped within porous
membranes of hollow spheres, while in extrusion the goal is to
entrap the active in a dense, impermeable glass. Encapsulating actives
via spray drying requires emulsifying the substrate into the
encapsulating agent. This is important for flavor applications, in
particular, considering the fact that most flavors are made up of
components of various chemistries (polarity, hydrophobic to hydrophilic
ratios), thus limiting their stability when dispersed or
suspended in different solvents. Hydrophobicity is one of the
most critical attributes that can play a significant role in determining
flavors’ payload as well as their release in food systems. The
basic principle of spray drying has been adequately covered by
[19].
Briefly, the process comprises atomizing a micronized (1–10-micron
droplet size) emulsion or suspension of an active and an encapsulating
substance and further spraying the same into a chamber.
Drying takes place at relatively high temperatures (210°C inlet
and 90°C outlet), though the emulsion’s exposure to these temperatures
lasts only for few seconds. The process results in free
flowing, low bulk density powders of 10–100-micron size. Optimal
payloads of 20% can be expected for flavors encapsulated in
starch matrices. Maltodextrins and sugars with lower molecular
weight, due to their low viscosities and inadequate emulsifying
activities, result in lower flavor payloads. Several factors can impact
the efficiency of encapsulation via spray drying, mainly those
related to the emulsion (solid content, molecular weight, emulsion
droplet size, and viscosity) and to the process (feed flow rate, inlet/
outlet temperature, gas velocity, and so on).
Release of flavors from spray-dried matrices takes place upon reconstitution
of the dried emulsion in the release medium, water
most often. Reasonable prediction of the release behavior should
take into consideration the complex chemistry of flavors and the
prevailing partition and phase transport mechanisms between
aqueous and non-aqueous phases [20, 21]. Encapsulation into an
amorphous matrix via extrusion has gained wide popularity with
applications ranging from entrapping flavors for their controlled
release to masking the grittiness of minerals and vitamins. Hot
melt extrusion is a highly integrated process with many unique
advantages for encapsulation applications, namely:
• Extruders are multifunctional systems (many unit operations)
that can be manipulated to provide desired processing temperature
and shear rate profiles by varying screw design, barrel heating,
mixing speed, feed rate, moisture content, plasticizers, and
so on.
• Possibility of incorporating actives and other ingredients at different
points of the extrusion process. Heat-labile actives, for
example, can be incorporated via temperature-controlled inlets
toward the end of the barrel and their residence time in the extruder
can be minimized to avoid degradation of the active and to
preserve its integrity.
• Extruders are also formers: encapsulated products can be recovered
in practically any a desired shape or size (pellets, rods, ropes,
and so on).
• Only very limited amount of water is needed to transform carbohydrates
from their native crystalline structure to amorphous
glassy matrices in an extruder, thus limiting the need for expensive
downstream drying.
• High payload: up to 30% can be expected when encapsulating
solid actives in extruded pellets.
• Economics: attributes such as high throughput, continuous
mode, and limited need for drying make extrusion a very attractive
process for manufacturing encapsulated ingredients.
Carbohydrate (encapsulating matrix), a mixture of sucrose and
maltodextrin, is dry fed and melted by a combination of heat
and shear in the extruder barrel so that the crystalline structure
is transformed into an amorphous phase. It should be cautioned
that although glass transition and associated microcapsule stability
are clearly related to the material properties of the matrix and
rates of crystallization, there is growing evidence that in the glass
transition region small molecules are more mobile than might be
expected from the high viscosity of the matrix [22].
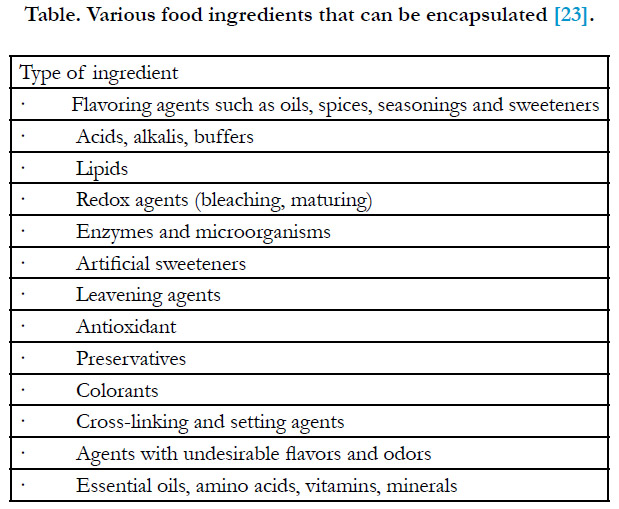
Types of Encapsulated Food Ingredients
The types of food ingredients that can be encapsulated are shown
in Table [23]. Applications for encapsulation have been slow to
expand since the technique was formerly thought to be too expensive
and highly specific. However, since production volumes
have increased and become more cost-effective, a wide variety of
encapsulated foods can be found. Flavored oil encapsulated in
food-grade hydrocolloid is an example of water-soluble capsules
commonly found.
Flavoring agents and spices are encapsulated by a variety of processes
and offer numerous advantages to the food processor. Citrus
oil and other flavors, for example, provide enhanced stability
to oxidation, volatilization, and light, controlled release, resistance
to clumping and caking, and substantially longer shelf life [24].
Encapsulated flavors are available as natural flavors, natural and
artificial flavors, essential oils (menthol, peppermint, and spearmint),
oleoresins, natural flavors with other natural flavors added,
chips, and artificial flavors. Although encapsulated flavors may
be used in many different applications, they are currently gaining
considerable attention for their stability through high-temperature/
short-time processes such as those utilized in preparing extruded
foods and microwavable foods.
Acidulants are added to foods as flavor modifiers, preservatives,
and processing aids. Unencapsulated food acids can react with
food ingredients to produce many undesirable effects. These include
deceased shelf life of citrus flavored foods and starch containing
foods, loss of flavor, degradation of color, and separation
of ingredients. Encapsulated food acids resolve these and other
problems because they preclude oxidation and provide controlled
release, with their coating formulated to dissolve or melt at specific
temperatures. Furthermore, encapsulated acids reduce hygroscopicity,
reduce dusting, and provide a high degree of flowability
without clumping. Examples of encapsulated acidulants that are
commercially available are adipic acid, ascorbic acid, citric acid,
fumaric acid, lactic acid, and malic acid [24]. Encapsulated acidulants
can be used as dough conditioners and in meat processing
(e.g., in cured meat products). For example, uncoated lactic acid
and citric acid cannot be used in the production of cured meats
because they react almost instantaneously with the meat, rendering
it unsuitable for further processing. However, an encapsulated
acid that is formulated for delayed release at smoldering temperatures
can be used, reproducing the same pH as that obtained
with lactic acid bacteria, eliminating the need for fermentation.
Thereby, production time can be reduced.
Microencapsulation also enables ingredients such as enzymes to
maintain their viability for extended periods of time, avoiding their
exposure to ions, protons, free radicals or other type of deleterious
agent. Sweeteners are often subject to the effects of moisture
and/or temperature. Encapsulation of sweeteners, namely sugars
and other nutritive sweeteners, reduces their hygroscopicity, improves
their flowability, and prolongs their sweetness perception.
Sodium chloride, encapsulated with a variety of coatings, including
partially hydrogenated vegetable oil, is used in formulations to
control color degradation, rancidity, water absorption, and yeast
growth. The encapsulated form also improves flowability and reduces
clumping and caking. Typical product applications include
ground meats, pretzel snacks, and yeast dough [24]. Leavening
agents such as sodium bicarbonate are used in baked goods to
achieve volume andlightness of texture. Encapsulated sodium bicarbonate
protects the base from premature reaction with acid or
water, and delays the release of its contents until optimum baking
conditions are present. This ensures that maximum leavening is
achieved and proves to be economically attractive.
Micro-organisms are the main agents responsible for food spoilage
and food poisoning and therefore food preservation procedures
are targeted towards them. Food preservation methods
currently used by the industry rely either on the inhibition of
microbial growth or on microbial inactivation. Methods which
prevent or slow down microbial growth cannot completely assure
food safety, as their efficacy depends on the environmental conditions.
Microbial can be inactivated through the treatment by heat,
chemical agents, radiations and the combination of these.
Inactivation by Heat
Heat has been widely used in the food industry as a preservation
agent, since it is capable of inactivating most microorganisms
and enzymes present in foods. Therefore, heat is a method that
can simultaneously guarantee food safety and food stability. Heat
treatments can be classified into two groups depending on their
intensity and their objective: pasteurization and sterilization treatments.
Pasteurization treatments aim to inactivate vegetative cells
of pathogenic species present in foods; they also extend shelf
life, as long as foods are maintained under refrigeration conditions.
Sterilization treatments are applied in order to guarantee
the stability of the food product at room temperature, an objective
that requires the application of temperatures above 100°C in
most cases. Such intense treatments are capable of inactivating
microbial spores as well as many enzymes and toxins present in
foods, but can also severely modify their organoleptic and nutritional
properties. Thermal treatments are widely used because of
their capacity to inactivate vegetative cells, bacterial spores, yeast
and molds. The type of inactivated microorganism depends, of
course, on the treatment’s intensity. The degree of heat resistance
of different microbial groups varies widely, due to their differing
structure and composition, as well as the mechanisms of resistance
they are able to develop. The application of this basic knowledge
could help improve the design of current pasteurization processes,
leading to milder and/or more effective treatments that
could fulfill consumer requirements for fresh-like foods while
maintaining the advantages of traditional heat treatments [25].
Inactivation by irradiation
Irradiation of foods and feeds for the purpose of killing indigenous
microbes, and thereby extending shelf life, has been recognized
as a preservation technique for several decades. Irradiation
also can be successfully applied to fresh fruits and vegetables for
the purpose of controlling disease and deterioration caused by
molds as well as for achieving insect disinfestation.
The survival of microbial cells upon treatment with irradiation
depends on several factors [26]. These include the nature and extent
of direct damage produced inside the vital target, the number,
nature, and longevity of irradiation-induced reactive chemical
species, and the inherent. ability of the cell to withstand these
assaults and undergo repair. Resistance also depends on extracellular
environmental conditions such as pH, temperature, and
chemical composition of the food in which cells are suspended.
Ionizing irradiation damages DNA at the cellular level, thus debilitating
normal biochemical processes.
Chemical inactivation
There are many chemicals that will kill or inhibit the growth of
microorganisms. An antibiotic generally refers to a chemical that
can be used on or inside a patient (humans, pets, livestock, etc.)
to inhibit the growth of microbes or kill microbes. Commonly
used chemical preservatives include sorbic acid, benzoic acid, and
propionic acid, and their more soluble salts potassium sorbate,
sodium benzoate, and calcium propionate, all of which are used
to control the growth of molds in acidic foods.
Microbial inactivation by irradiation, ultrasound under pressure,
HHP and PEF has been found to depend on many factors. Effective
comparison of data published in literature is hampered by the
diversity of equipment’s and experimental conditions employed
by the different authors. Nevertheless, this section tries to give an
overview on the most relevant factors affecting resistance to novel
technologies. The factors are classified into three groups: process
parameters, microbial characteristics and product parameters.
Process parameters
Some process parameters are intrinsic to each technology and no
general conclusions can be drawn. For instance, the intensity of
an irradiation treatment is given by the irradiation dose absorbed,
as the radiation energy is normally fixed [25]. Critical inherent
parameters for ultrasound under pressure are treatment time, amplitude
of the ultrasonic waves and external pressure applied [26].
Microbial characteristic
Maximum inactivation levels attained with each technology will
depend on factors such as equipment technical developments and
food characteristics. The point is that comparison of data is compered
by the different equipment’s, treatment media, strains, etc.
Microbial resistance to different physical agents depends not only
on the intrinsic resistance of the micro-organisms but also on
their physiological state. It is well known that bacterial heat resistance varies widely depending on the growth phase, growth temperature
and exposure to previous stressing environments [27].
Product parameters
Environmental factors, such as composition of the treatment medium,
pH, water activity or addition of preservative substances,
strongly affect the resistance of micro-organisms to heat. The
relative influence of such factors on microbial resistance to novel
technologies depends on their mechanisms of action. One of
the most important factors influencing irradiation sensitivity is
the composition of the treatment atmosphere. The presence of
oxygen during irradiation has been found to enhance lethal effect
because of oxygen radical formation [25].
Microwave Combination Technology
food products heated by MW shows better retention in color, texture,
and flavor compared with conventionally treated products,
MW heating is associated with numerous problems, such as nonuniform
heating, partial overheating, and limited penetration [28,
29]. Conventional methods such as vacuum drying (VC) and hot
air (HA) heating can preserve the quality of perishable agricultural
products without any damage during processing; however,
it takes considerable time and consumes more energy with low
energy efficiency to complete the processing [30]. MW technology
combined with the aforementioned conventional methods
has been investigated particularly in drying and baking processes.
Infrared Radiation Combination Technology
IR heating is considered a promising method especially for drying
processes, observed problems in IR drying include scorching
heat on the surface of food products and a limited IR penetration
depth [31]. Case hardening is a troublesome problem occurring
in conventional HA drying process because the surface
of food material is dried first, and as drying process progress,
the dried surface of food becomes a barrier to heat transfer [32].
To prevent undesirable phenomenon caused by either IR or conventional
heating methods, a number of studies on dehydration
of food products using integrated IR and conventional methods
have been conducted.IR-assisted HA drying processes for fruit
and vegetable has been evaluated and developed [33, 34].
High-Pressure Processing Combination Technology
High-pressure processing (HPP) has been mainly applied to pasteurize
liquid food products; however, it often times could not
inactivate bacterial spores (e.g., Bacillus and Salmonella) which are
heat and acidic resistant [35]. Therefore, thermal treatment has
been applied to HPP as a pretreatment step. The effectiveness
of HPP combined with thermal treatment (TH) on the inactivation
of PMEs and the inactivation kinetics in various agricultural
products were evaluated by a number of researchers [36].
Radio Frequency Electric Field Combination Technology:
Ukuku and Geveke [37] developed a combined UV light and RF
electric field (RFEF) system to inactivate Escherichia coli K-12
in apple juice. Apple juice was preheated up to 25, 30, and 40 0C
and then treated by individual UV, RF and combined UV with
RF treatment. After all treatments, apple juice samples inoculated
with microbial contaminant were analyzed for leakage of UVabsorbing
substances as the function of cell membrane injury.
The individual UV and RFEF treatment at 400C showed the minimum
surviving population of E. coli K-12 in the juice. A higher
bacterial inactivation was expected when the two treatments were
combined; however, the determined number was only an approximately
0.6 log microbial reduction higher than UV treatment
alone. Although inactivation of E. coli K-12 in apple juice was not
influenced by the combination system, UV-absorbing substances
determined in the juice treated by combined treatment was substantially
different from individual UV treated sample. The results
suggested that combination treatment would damage bacterial
cells and lead to more leakage of intracellular UV-absorbing substances
than individual treatment.
Combined RF with HA treatment was investigated to improve
the quality and mold control of enriched white bread [38]. Prior
to RF–HA treatment, the bread columns inoculated with mold
spores were kept under a sterile hood in order to equilibrate moisture
content in the breads. Additionally, target HA and treatment
temperatures controlled by an electrical fan heater and RF power
were evaluated to maximize the mold lethal condition. Visible
mold growth was observed from the surface of untreated bread
loaves stored for five weeks at room temperature; on the other
hand, mold was found in the sample after an extra four weeks
using the combined RF–HA treatment. Moisture migration from
the bread crumb to crust was caused by generation of internal
vapor pressure during the RF heating. The consequent moisture
loss in the bread crumb and increased moisture at the crust led to
a more even distribution of moisture in the treated bread samples.
Combined RF and HA treatment had little effect on the water
activity of breads during storage.
Pulsed Electric Field combination technology: Synergistic effect
of combined thermal treatment (TH) and pulsed electric field
(PEF) on inactivation of microorganisms in liquid food products
has been investigated by a number of researchers [39, 40]. In
these studies, liquid food products (such as salad dressing, liquid
whole egg, liquid egg york, apple juice, fruit smoothie-type beverage)
pretreated using a heat exchanger, heating coil, or hot water
bath at different temperatures were sequentially applied to the
pulsed electric field (PEF) treatment. The effect of sequential TH
and PEF treatment on inactivation of microbial contaminants,
i.e., Lactobacillus plantarum, Escherichia coli O157:H7, Salmonella
enteritidis in respective salad dressing, liquid whole egg, and
liquid egg yolk was also investigated [39, 40]. Prior to PEF treatment,
the liquid food product was preheated up to a certain temperature
in the hot water bath. Preheated sample flowed between
two disk electrodes and then through an electric field with a range
of 9–15 kV/m with different pulse numbers and high frequency.
The pulse width and frequency were adjusted using external transistor–
transistor logic (TTL) with a frequency trigger. Increasing
the pretreatment temperature of liquid food product (apple juice
and liquid egg yolk) and higher electric field strength had a significant
effect on the inactivation of peroxidase (POD), polyphenol
oxidase (PPO), and E. coli O157, as well as, lower D-values [39].
Ohmic Heating Combination Technology: Combined ohmic
and plate heating system for cooking hamburger patties was developed for the enhancement of physical properties of the patties
[41]. A domestic plate grill was modified for the combination
system. The plate was preheated first and then 50 V of alternating
current was applied for OH. The required cooking time was determined
to be 117 and 163 s for the combined and conventional
techniques, respectively. The elasticity index of the conventionally
cooked meat has a slightly higher value than that of cooked meat
by ohmic–plate heating. This suggested that the meat cooked by
the combination system would be less chewy. Otherwise, the mechanical
properties of the meats cooked by individual plate and
OH methods were very similar. The application of OH for cooking
of hamburger patties did not affect the taste and texture of
the meat.
Encapsulation technology has been used in various industries for
more than seven decades, there have been several advancements
in both the science as well as the practical application of this technique
since its first commercial application in 1950. It is being increasingly
popular in pharmaceutical, nutraceutical and functional
food industries as a highly effective method that performs various
functions; the major being prolonging the shelf-life of the active,
masking the undesirable flavour, colour and taste and controlling
the release of bioactive. Encapsulation methods for new bio-actives
are being explored and research advancement is underway to
improve the process and product characteristics.
Innovative food-grade encapsulants are being explored to reduce
the production costs and meet other technical specifications and
consumer expectations. With the escalating demand of functional
foods including omega-3s, probiotics, vitamins and phytochemicals,
these functional ingredients are being incorporated into
wide range of products such as breads, milk, fruit juices, tortillas,
chocolate, yoghurt drinks, spreads, peanut butter, eggs and meat.
Accordingly, various methods of microencapsulation of different
bioactives have been developed. At present, spray drying-based
microencapsulation method is being widely used in various industrial
applications; however, more advanced methods including
complex coacervation are gaining increased attention in recent
years. Complex coacervation technology has been reported to
receive a high product yield and the resultant product possesses
prolonged stability even at a very high payload (up to 99%). In addition,
it yields products with lowest unit product cost [43]. The
biggest disadvantage of this technology is limited availability of
shell materials. So far, gelatin is the only protein which is successfully
used in commercial scale.
A number of studied have reported that the plant proteins are capable
of forming coacervates in the presence of polysaccharides
[44, 45]. This corroborates that plant proteins can be used instead
of animal proteins in complex coacervation process. Reference
[46] used α-gliadin (cereals) and pea globulin (legume) in complex
coacervation process. These authors found that both these proteins
form excellent complex coacervates with the gum Arabic.
However, the application of α-gliadin in the coacervation process
will not achieve widespread acceptance as this protein is associated
with some kind of allergenicity in some individuals [46]. So,
there is a need to test other plant polysaccharides for their potential
as encapsulating and delivery vehicles of active ingredients.
There are certain characteristics which are looked for before using
a biopolymer as an encapsulant. Among them are emulsifying
and interfacial properties, film forming abilities, solubility and gelforming
properties. Emulsifying properties of flaxseed protein,
chia seed protein and lentil protein have been evaluated in recent
years [44, 47, 48]. It was found that emulsions stabilized by flax
protein concentrate (FPC) at neutral pH and in the absence of
salt had a smaller droplet size and higher surface charge which
makes them good candidates to be used in coacervation process.
FPC-stabilized emulsions were more stable against the effect of
salt concentration.
The FPC can be effective stabilizing emulsions where droplet size
and zeta-potential are major factors influencing the emulsion stability.
Flaxseed gum is also found to possess good potential in
stabilizing the protein-based emulsions. Encapsulating unstable
and bioactive core materials with a protein-gum complex shell
matrix isolated from the same plant source is a very recent idea
of microencapsulation. Reference [47] successfully encapsulated
flaxseed oil (core) by novel matrix of flaxseed protein-flaxseed
gum complex coacervate. Similarly, [44] successfully encapsulated
chia seed oil using chia seed protein-gum complex coacervate
shell matrix. The authors have compared the effectiveness of protein
only and gum only shell matrix with the complex coacervate
shell matrix and concluded that complex coacervation based shell
matrix is more effective over the other two. However, this laboratory
experiments need further study for their effectiveness and
reproducibility in pilot plant or commercial trials.
Conclusion
There are various reasons of encapsulation, many bioactive ingredients
are encapsulated to enhance their longevity and functionality.
Several bioactive ingredients are encapsulated to prevent
their degradation from environmental stressors and control their
release in the gastrointestinal tract. For example, baking yeast and
dough conditioners are encapsulated to increase their performance
or to overcome other processing challenges. It has been
reported that uncoated chemical leaveners release carbon dioxide
prematurely. This is even more prominent in warmer environments.
In addition, ingredient degradation or flavour loss during
the baking process can occur in systems where uncoated ingredients
are used. For instance, PUFAs-rich oils are encapsulated to
prevent or minimize their oxidation. Bioactive peptides are encapsulated
to control their release in targeted site. Therefore, encapsulation
method is dependent on the nature of core material
and intended use of the final product. As a consequence, various
methods of encapsulation are developed.
References
-
[1]. Zuidam NJ, Nedovic V. Encapsulation technologies for active food ingredients
and food processing. 2010.
[2]. Fang Z, Bhandari B. Encapsulation of polyphenols–a review. Trends in Food Science & Technology. 2010 Oct 1; 21(10): 510-23.
[3]. de Vos P, Faas MM, Spasojevic M, Sikkema J. Encapsulation for preservation of functionality and targeted delivery of bioactive food components. International dairy journal. 2010 Apr 1; 20(4): 292-302.
[4]. Desai KG, Jin Park H. Recent developments in microencapsulation of food ingredients.Drying technology. 2005 Jul 1; 23(7): 1361-94.
[5]. Lesmes U, McClements DJ. Structure–function relationships to guide rational design and fabrication of particulate food delivery systems.Trends in Food Science & Technology. 2009 Oct 1;20(10):448-57.
[6]. King W, Perry P. Modified starch encapsulating agents offer superior emulsification, film forming, and low surface oil [Incorporation into dry food systems].Food Product Development. 1976.
[7]. Drusch S, Serfert Y, Schwarz K. Microencapsulation of fish oil with n‐octenylsuccinate‐ derivatised starch: Flow properties and oxidative stability. European Journal of Lipid Science and Technology. 2006 Jun;108(6):501-12.
[8]. Jeon YJ, Vasanthan T, Temelli F, Song BK. The suitability of barley and corn starches in their native and chemically modified forms for volatile meat flavor encapsulation. Food Research International. 2003 Jan 1;36(4):349-55.
[9]. Trubiano PC,Lacourse NL. Emulsion-Stabilising Starches: Use in Flavor. In: Risch SJ and Reineccius, GA., Eds., Flavor Encapsulation, American Chemical Society, Washington. 1988. 45-54.
[10]. Bylaitë E, Venskutonis PR, Maþdþierienë R. Properties of caraway (Carumcarvi L.) essential oil encapsulated into milk protein-based matrices. European Food Research and Technology. 2001 Jun;212(6):661-70.
[11]. Li JK, Wang N, Wu XS. Gelatin nanoencapsulation of protein/peptide drugs using an emulsifier-free emulsion method.J Microencapsul. 1998 Mar- Apr;15(2):163-72. PMID: 9532522.
[12]. Kim YD, Morr CV, Schenz TW. Microencapsulation properties of gum arabic and several food proteins: liquid orange oil emulsion particles. Journal of Agricultural and Food Chemistry. 1996 May 16; 44(5): 1308-13.
[13]. Jimenez M, Garcia HS, Beristain CI. Spray-drying microencapsulation and oxidative stability of conjugated linoleic acid.European Food Research and Technology. 2004 Nov;219(6):588-92.
[14]. Damodaran S. Protein stabilization of emulsions and foams. Journal of Food Science. 2005 Apr;70(3):R54-66.
[15]. Sheu TY, Rosenberg M. Microstructure of microcapsules consisting of whey proteins and carbohydrates. Journal of Food Science. 1998 May;63(3):491- 4.
[16]. Keogh MK, O'kennedy BT, Kelly J, Auty MA, Kelly PM, Fureby A, et al. Stability to oxidation of spray‐dried fish oil powder microencapsulated using milk ingredients. Journal of Food Science. 2001 Mar;66(2):217-24.
[17]. Fathi M, Mozafari MR, Mohebbi M. Nanoencapsulation of food ingredients using lipid based delivery systems. Trends in food science & technology. 2012 Jan 1;23(1):13-27.
[18]. Conde-Petit B, Escher F, Nuessli J. Structural features of starch-flavor complexation in food model systems. Trends in food science & technology. 2006 May 1; 17(5): 227-35.
[19]. Masters K. Spray drying handbook. Spray drying handbook. 1985. [20]. Labrousse S, Roos Y, Karel M. Collapse and crystallization in amorphous matrices with encapsulated compounds. Sciences des aliments. 1992;12(4):757-69.
[21]. Shimada Y, Roos Y, Karel M. Oxidation of methyl linoleate encapsulated in amorphous lactose-based food model. J Agric Food Chem. 1991 Apr;39(4):637-41.
[22]. Parker R, Ring SG. Diffusion in maltose-water mixtures at temperatures close to the glass transition.CarbohydrRes. 1995 Aug 25;273(2):147-55. [23]. F. Gibbs, SelimKermasha, InteazAlli, Catherine N. Mulligan B. Encapsulation in the food industry: a review. Int J Food SciNutr. 1999 Jan 1;50(3):213-24.
[24]. Shahidi F, Han XQ. Encapsulation of food ingredients.Crit Rev Food Sci- Human Nutr. 1993 Jan 1;33(6):501-47.
[25]. Gould GW. Heat-induced injury and inactivation.Mechanisms of action of food preservation procedures. 1989:11-42.
[26]. Farkas J. Microbiological safety of irradiated foods. Int J Food Microbiol. 1989 Aug 1;9(1):1-5.
[27]. Gentry TS, Roberts JS. Design and evaluation of a continuous flow microwave pasteurization system for apple cider. LWT-Food Science and Technology. 2005 May 1;38(3):227-38.
[28]. Nguyen LT, Choi W, Lee SH, Jun S. Exploring the heating patterns of multiphase foods in a continuous flow, simultaneous microwave and ohmic combination heater. J Food Eng. 2013 May 1;116(1):65-71.
[29]. Varith J, Noochuay C, Netsawang P, Hirunstitporn B, Janin S, Krairiksh M (2007) Design of multimode-circular microwave cavity for agri-food processing. In: Microwave conference, 2007. APMC 2007. Asia-Pacific IEEE. pp 1–4.
[30]. Sakai N, Hanzawa T. Heat Transfer Analysis in Food Heated by Far-Infrared Radiation. Developments in Food Engineering.Springer USA; 1994.pp 313–315.
[31]. Ratti C. Shrinkage during drying of foodstuffs. J Food Eng. 1994 Jan 1;23(1):91-105.
[32]. Afzal TM, Abe T, Hikida Y. Energy and quality aspects during combined FIR-convection drying of barley. J Food Eng. 1999 Dec 1;42(4):177-82.
[33]. Hebbar HU, Vishwanathan KH, Ramesh MN. Development of combined infrared and hot air dryer for vegetables.J Food Eng. 2004 Dec 1;65(4):557- 63.
[34]. Krebbers B, Matser AM, Hoogerwerf SW, Moezelaar R, Tomassen MM, van den Berg RW. Combined high-pressure and thermal treatments for processing of tomato puree: evaluation of microbial inactivation and quality parameters. Innov Food SciEmerg. 2003 Dec 1;4(4):377-85.
[35]. Castro SM, Loey AV, Saraiva JA, Smout C, Hendrickx M. Inactivation of pepper (Capsicum annuum) pectin methylesterase by combined high-pressure and temperature treatments. J Food Eng. 2006;75(1):50–58.
[36]. Ukuku DO, Geveke DJ. A combined treatment of UV-light and radio frequency electric field for the inactivation of Escherichia coli K-12 in apple juice.Int J Food Microbiol. 2010 Mar 31;138(1-2):50-5.
[37]. Liu Y, Tang J, Mao Z, Mah JH, Jiao S, Wang S. Quality and mold control of enriched white bread by combined radio frequency and hot air treatment. J Food Eng. 2011 Jun 1;104(4):492-8.
[38]. Amiali M, Ngadi MO, Smith JP, Raghavan GS. Synergistic effect of temperature and pulsed electric field on inactivation of Escherichia coli O157: H7 and Salmonella enteritidis in liquid egg yolk. J Food Eng. 2007 Mar 1;79(2):689-94.
[39]. Bazhal MI, Ngadi MO, Raghavan GS, Smith JP. Inactivation of Escherichia coli O157: H7 in liquid whole egg using combined pulsed electric field and thermal treatments. LWT Food Sci Technol. 2006 May 1;39(4):420-6.
[40]. Gin B, Farid M. The use of carbon electrodes in ohmic cooking of meat patties. Asia-Pacific J Chem Eng. 2007 Sep;2(5):474-9.
[41]. Erkmen O, Bozoglu TF. Food Microbiology, 2 Volume Set: Principles into Practice. John Wiley & Sons; 2016 Jun 13.
[42]. Barrow CJ, Nolan C, Jin Y. Stabilization of highly unsaturated fatty acids and delivery into foods. Lipid Technology. 2007 May;19(5):108-11.
[43]. Timilsena YP, Wang B, Adhikari R, Adhikari B. Preparation and characterization of chia seed protein isolate–chia seed gum complex coacervates. Food hydrocolloids. 2016 Jan 1;52:554-63.
[44]. Kaushik P, Dowling K, Barrow CJ, Adhikari B. Complex coacervation between flaxseed protein isolate and flaxseed gum. Food Research International. 2015 Jun 1;72:91-7.
[45]. Ducel V, Richard J, Popineau Y, Boury F. Adsorption kinetics and rheological interfacial properties of plant proteins at the oil− water interface. Biomacromolecules. 2004 Nov 8;5(6):2088-93.
[46]. Kaushik P, Dowling K, McKnight S, Barrow CJ, Adhikari B. Microencapsulation of flaxseed oil in flaxseed protein and flaxseed gum complex coacervates. Food research international. 2016 Aug 1;86:1-8.
[47]. Wang B, Li D, Wang LJ, Adhikari B, Shi J. Ability of flaxseed and soybean protein concentrates to stabilize oil-in-water emulsions. Journal of Food Engineering. 2010 Oct 1;100(3):417-26.
[48]. Wang B, Wang LJ, Li D, Adhikari B, Shi J. Effect of gum Arabic on stability of oil-in-water emulsion stabilized by flaxseed and soybean protein. Carbohydrate polymers. 2011 Aug 1;86(1):343-51.