Combination of Autosomal and Y-STR Analysis, an Alternative to Differential Extraction: A Case Study
Sandip Ghosh1*, Sutanwi Bhuiya2#, Bipradut Sil3#
1 Assistant Director, Biology Division, Forensic Science laboratory (Biology Division), Kolkata, Government of West Bengal, West Bengal, India.
2 Forensic Science Laboratory (Biology Division), Kolkata, Government of West Bengal, West Bengal, India.
3 Forensic Science Laboratory (Biology Division), Kolkata, Government of West Bengal, West Bengal, India.
# Both authors have equally contributed in this study.
*Corresponding Author
Sandip Ghosh,
Assistant Director, Biology Division, Forensic Science laboratory (Biology Division), Kolkata, Government of West Bengal, West Bengal, India.
E-mail: sandip@rocketmail.com
Received: December 14, 2021; Accepted: January 18, 2022; Published: January 24, 2022
Citation: Sandip Ghosh, Sutanwi Bhuiya, Bipradut Sil. Combination of Autosomal and Y-STR Analysis, an Alternative to Differential Extraction: A Case Study. Int J Forensic Sci Pathol. 2021;9(1):468-474. doi: dx.doi.org/10.19070/2332-287X-2200098
Copyright: Sandip Ghosh©2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The samples from the sexual assault cases are often found as a imbalanced mixture of epithelial cells of victim and accused sperms, with an excess of victim’s material resulting in an unfavorable ratio of male to female. As the male-DNA contribution is much lower than the female epithelial cell population in gynecological sample, analysis and interpretation of the individual contributions, has always been a challenge for the forensic scientists. Various techniques have been tried to separate the male DNA from the female part, including Differential Extraction (DE), but none of those are cost effective and time saving. The Y-STR haplo-typing, has been used for the mixed sample where the autosomal STR failed to produce visible peaks for the male DNA. In those cases combined autosomal and Y-STR analysis provided additional leads for the investigation. Here we report one case, where we conducted both autosomal-STR and Y-STR analysis, in combination, from the gynecological mixture sample, completely avoiding the complex ‘Differential Extraction’. The male to female contribution ratio in a gynecological sample, was found to be 1: 3.39 as deduced by the real-time qPCR using QuantiFilerTM Trio kit. As the mixed autosomal profile included both the suspect and the victim , using the qualitative and semi-quantitative binary model of interpretation, the Y-STR analysis confirmed the offender. This method is applicable when a preliminary suspect list is available to the investigative agency to compare with. Since this method is simple and is not influenced either by the azoospermic and vasectomized male samples or mixed samples other than semen viz. male DNA under fingernails of the victim, male touch DNA on the skin of the victim, and the clothing or belongings of a female victim, we propose this combination method may be highly effective in resolving a broad spectrum of cases in the Forensic laboratory.
2.Keywords
3.Introduction
4.Methodology
5.Case Report
6.Discussion
7.Conclusion
8.References
Keywords
Sexual Assault Cases, Differential Extraction; Autosomal–STR Analysis; Y-STR Analysis; qPCR; Combination of Autosomal & Y-STR Analysis by Capillary Electrophoresis.
Introduction
For the last 3-4 yrs, the pendency of the sexual assault cases has
become a serious concern to the Court of Law, all over the globe.
The failure to investigate those cases, in a time dependent manner,
creates a huge problems for victims, public safety and thereby
causes a serious hurdle in criminal justice system. In cases of sexual
assault, if traces of semen are left behind, either on the victims
cloth or in place of occurrence, then semen detection becomes
instrumental to solve the puzzle. Moreover, there are cases where
semen are mixed with victims sample (gynecological samples) and
in such a case, the challenge becomes two fold. First, is to detect
the semen as it depends on a lot of factors, such as, presence/
absence of the ejaculation, time elapsed between time of crime
and sample collection and storage of the samples etc [1-3] and the
second, is to separate the male spermatozoa from the mixed stain
for following Y-STR analysis to identify the male contributor(s).
In many cases, DNA analysis of the offender becomes extremely
difficult due to the presence of the high female material apart
from the analytical method itself [4-6]. Three studies [7-9] have
used the preferential lysis method to separate male part from the
mixed stain and typed the DNA by Southern Hybridization [10].
That method was modified by Yoshida et al, 1995 [11] where
they used a two step differential extraction to enrich the spermDNA part which is now mostly used by different laboratories all
over the world, with minor variations and is considered to be the
“Gold Standard’ for mixed gynecological samples [12].
However, the methods are laborious, skill-dependent and are not
always successful in achieving complete separation of the two
cellular fractions. It often failed in cases with very small and degraded
samples [13] and little sperm sample could be available for
downstream DNA profiling by Y-STR typing, although Y-specific
amelogenin could be detected in most of them. Incidentally, one
study showed that sample with low sperm counts were misclassified
as sperm –free in 73% of the samples tested [14] Another
study indicated that a 90% loss in male-DNA, initially present in
simulated sexual-assault samples [15]. Moreover, it is time consuming,
and requires extensive sample handling and difficult to
automate [5, 15, 16]. To circumvent the conventional DE, various
rapid techniques including automated methods for differential
separation of sperm cells have been developed, such as, Laser
Micro-dissection [17-20], which allows the excision and collection
of spermatozoa independent of surrounding cellular material,
differential lysis [21] in combination with sperm elution [22],
Membrane Filtration and micro-devices that exploit differences
between size and shape of the cells [23-27], Flow-cytometry,
which takes advantage of specific membrane protein to mark and
sort cells [28], Alkaline plate based methods [29], Antibody based
cell culture [30-33], Enzymatic digestion [34] etc, .
Unfortunately, all of these methods are either too costly (flow
cytometry), or low throughput (Micro-dissection) or prone to be
non-functional as a result of loss of antigen specificity in old samples
or degradation of antibody (for antibody-based separation).
Autosomal STR analysis for the mixed samples has been used
by various laboratories mostly due to its individualizing capacity
and the existence of national DNA databases of standardized
STR profiles from autosomal alone. Nevertheless, Y-haplotyping,
is shown to be useful [35], to get informative result, in those cases
where autosomal tests are limited by the evidence, such as high
levels of female DNA in the presence of minor amounts of male
DNA (sperm-DNA count is less than one in 20 of female-DNA)
[36, 37], sexual assault evidences from azoospermic or vasectomized
males and blood–blood or saliva–blood mixtures where
the absence of sperm prevents the differential extraction for isolation
of male DNA [38, 39]. In addition, the number of individuals
involved in a “gang rape” may be easier to decipher with
Y-chromosome results than with highly complicated autosomal
STR mixtures . Using Chromosome Y-specific PCR primers thus
can improve the chances of detecting low levels of the perpetrator’s
DNA in a high background of a female DNA [40-44].
Y-chromosome tests have also been used to verify amelogenin
Y-deficient males [45].
However, Y-STR profile, by itself, is not as informative as an
autosomal STR profile. That is mainly because (1) paternally
related males cannot be discriminated, since they have identical
Y-chromosome [46], and thus Y-STR typing cannot be used to
distinguish brothers or even distant paternal relatives and (2) the
frequency of a specific Y-STR profile in the population can be
relatively high [47], impeding the discrimination of some unrelated
males. A combination method of autosomal and Y-STR has
been validated where it was shown as a additional investigative
lead for the prosecution [48].
Since the last few years the load of sexual assault cases has increased
alarmingly in India, which requires a quick scientific response
to convict the offender without compromising with the
merit of the cases. The method, we described, is lot easier and
can be performed within a considerable time frame, which completely
avoids complex DE methods. In addition, the limited absolute
quantity of the male contributory part is always a problem
in sexual assault cases. In such cases it is always advantageous to
use our method over the traditional separation technique, where
a considerable amount of the sperm cell is lost in separation process.
Moreover, as this combination method is independent of
the presence of spermatozoa in the seminal stain, it can be useful
for azoospermic or vasectomized male contributor also. The
method can be applicable for a mixtures of body fluids, such as
blood–blood or blood–saliva etc. as well. The process can utilize
any type of cells, other than spermatozoa, to get the contributory
profile. This way it has a vast potential to apply literary in any
types of forensic cases, where mixed samples are often encountered.
Here, we report, one sexual assault case, where a police case had
been filed in one of the rural districts of West Bengal, India. The
police had arrested two suspects, from whom the hair samples
were collected maintaining all legal formalities, along with the PM
blood and the gynecological sample collected by the doctor from
the inner side of the Victim’s Labia Majora using a cotton swab.
DNA extraction and real-time qPCR analysis suggested the male
to female ratio to be 1 : 3.39. The total genomic DNA was 2
ng/ul out of which the male DNA was 0.45 ng/ul in quantity
in the mixed gynecological sample. Due to limited male sample
we avoided the separation step to male from the female part as
it would have resulted a further loss of the male part, instead
direct autosomal STR followed by Y-STR typing was performed.
The expected mixed profile for autosomal-STR could be interpreted
qualitatively based upon the alleles present [49, 50], which
included the suspect along with the victim. The following Y-STR
analysis, then confirmed the offender, and thereby, led to exoneration
of the innocent male subject. In addition, the Amelogenin
locus has been targeted for amplification both in qPCR [51] and
in end point PCR assay [52]. We have showed almost identical X
to Y peak-height ratio for both real-time qPCR ( 7.78 : 1) using
QuantiFilerTM Trio Kit ( Applied Biosystems by ThermoFisher
Scientific) & end point PCR (8.08 : 1) using GlobalFilerTM kit (
Applied Biosystems by ThermoFisher Scientific). So we propose
our method to be extremely useful to individualize the perpetrator
in broad spectrum of forensic cases including the sexual assault,
where the male DNA is extremely small, but its contribution is
one in five of female part or more in the mixture.
Material and Methods
After the complaint lodged in a sexual assault, in one of the rural
districts of West Bengal, India, the gynecological sample, in this
case, a swab in cotton wool from the inner Labia Majora of the
deceased lady (A), P. M. Blood sample (B) and the hair samples
of the two suspects (S1 & S2) have been collected by the competent
Medical professional, complying with all legal formalities and
handed over to the police. Police has deposited the samples in the
laboratory eventually for DNA analysis. Samples were stored at
4°C as per standard protocol to reduce the levels of degradation.
The examination has been done after a fortnight of receiving in the laboratory.
The presence of semen in the Gynecological swab (A) was first
detected by presumptive acid-phosphatase test [53, 54] which was
confirmed by ABAcard® p30 (Abacus Diagnostics, Inc., West
Hills, CA) Kit as per manufacturer’s protocol using the prostate
specific antigen (PSA) activity in semen stain [55].
DNA extraction
The swab in cotton wool (gynecological swab and PM blood) and
the hair samples of the suspects (S1 & S2), were subjected to
DNA analysis. The cotton wool, cut into small pieces and 2-3 hair
samples with root from the suspects (S1 & S2) have been taken in
1.5 ml microfuge tube for analysis. Extraction of DNA was performed
in AutoMate Express™ Instrument (Applied Biosystems
by ThermoFisher Scientific) [56, 57]. Extraction of each sample
was performed in duplicate following the validation Guidelines issued
by the Scientific Working Group on DNA Analysis Methods
(SWGDAM). The purpose of this method was to extract the total
nuclear DNA from all the samples.
Quantification
After isolation, DNA samples were used for quantification using
Quantifiler™ Trio kit (Applied Bio-system by ThermoFisher
Scientific, USA) on Quant-Studio 5 following manufacturer’s protocol.
The assay combined four 5' nuclease (Taqman) assays [58];
two separate target-specific human assays; one with a short PCR
amplicon and one with a long PCR amplicon, a target-specific
human male DNA assay and an internal PCR control (IPC) assay.
Each target assay consisted of PCR primers and dye-labeled
TaqMan® probes with non-fluorescent quenchers for the amplification
of multi-copy genomic loci. Quantification DNA standards
were used to quantify the extracted DNA samples using HID
Real-Time PCR Analysis Software v1.3.
Amplification
After quantification, 1 ng of each of the extracted samples were
amplified using the GlobalFiler™ and Yfiler™ Plus amplification
kit (Applied Biosystems by ThermoFisher Scientific) as per manufacturer’s
protocol. The GlobalFiler™ amplification Kit consisted
of 6-dye based short tandem repeat (STR) multiplex assay for
the amplification of human genomic DNA. The kit amplified 24
STR loci including Amelogenin (sex determining marker). The
collection of STR analysis and Amelogenin amplicons together
constituted the DNA profile of the sample under examination.
The Yfiler™ Plus PCR Amplification Kit is a 6-dye, multiplex
(27 Y-STR ) assay optimized to allow amplification from multiple
male specific sample types such as male-male, male-female mixtures.
“DNA Control 007”, supplied in the kit- which was used
as a positive control for evaluating the efficiency of the amplification
step and genotyping for both autosomal and Y-STR. The
GlobalFiler™ Allelic Ladder for STR genotyping for accurate
characterization of the alleles for autosomal analysis & Yfiler™
Plus Allelic Ladder for Y-STR analysis were used.
Capillary electrophoresis
The fluorescently tagged DNA fragments which were then separated
by capillary electrophoresis (CE) and size-classified through
valuation with an internal standard on a Applied Biosystems 3500
Genetic Analyser (ThermoFisher Scientific, USA) using Data
Collection software v4.0.1 in Windows 10 platform. 1 µL of
sample (PCR product) was added to 9.6 µL Hi Di™ formamide
(ThermoFisher Scientific) and 0.4 µL GeneScan™ 600 LIZ™
Size Standard v2.0 (ThermoFisher Scientific). The CE run condition
was as per manufacturer’s protocol, which included POP-4
polymer (ThermoFisher Scientific), a 36 capillary array ( ThermoFisher
Scientific) and a 10s 3kV injection. Electropherogram
was analyzed using the GeneMapper IDX software v1.6 (Thermofisher
Scientific, MA, USA) by comparing the results to reference
allelic ladders.
Result and Discussion
The primary goal of this case study was to analyze both autosomal-
STR and Y-STR pattern(s) of the mixed male/female sample,
without separating the female epithelial cells from the male semen
fraction, to exclude and/or include the suspects. We initially detected
the presence of semen in the mixed sample by acid phosphatase
test and further confirmed it by showing the presence
of p30- antigen in the sample using Abacard p30-kit. The total
un-fractionated DNA from the swab taken from inner-side of the
Labia Majora (A) of the victim, from the hair sample of both the
suspects (S1 & S2) and victim’s PM blood (B), was extracted, and
quantified by qPCR using QuantiFiler Trio Kit (Applied Biosystems
by Thermo Fisher Scientific, USA). The female-male DNA
ratio was found to be 3.39 : 1 ( X : Y = 7.78 : 1) in the gynecological
sample by real-time qPCR. The autosomal STR analysis, for
all the samples, was then performed by capillary electrophoresis
in ABI 3500 and the data was analyzed using ID-X software, version
1.6.
The profiles using 21 autosomal STR-loci, Amel, Y-Indel and
DYS391, as supplied in GlobalFilerTM plus kit (Appled Biosystems
by ThermoFisher Scientific) was analyzed in the extracted
DNA-from the all the samples, i.e. the gynecological swab sample
of the victim (A), the victim’s PM blood (B), and the suspects
samples (S1 & S2) and the result obtained for 17 loci including
DYS391 has been represented in Figure 1. All the profiles for the
un-mixed samples (B, S1 & S2) were well balanced with heterozygous
peak height > 70% and minimum noise level. The gynecological
swab (A) showed a mixed profile with a maximum of four
(4) fluorescence peaks. The profile could be clearly differentiated
into two distinct components (Fig 1). The major profile was of
the victim (female) and the minor component had a male genotype
which matched exactly with one of the suspects (S1), except
for loci D2S1338, where one of the allele-peak (19) for the male
profile was higher. That may be due to incomplete or partial amplification
at that locus for the female part. The allele peak heights
for the male suspect were sometimes quite low, but visible in the
mixture (A). This is expected, because of preferential amplification
of the abundant female DNA template, as compared to the
male part in the mixture.
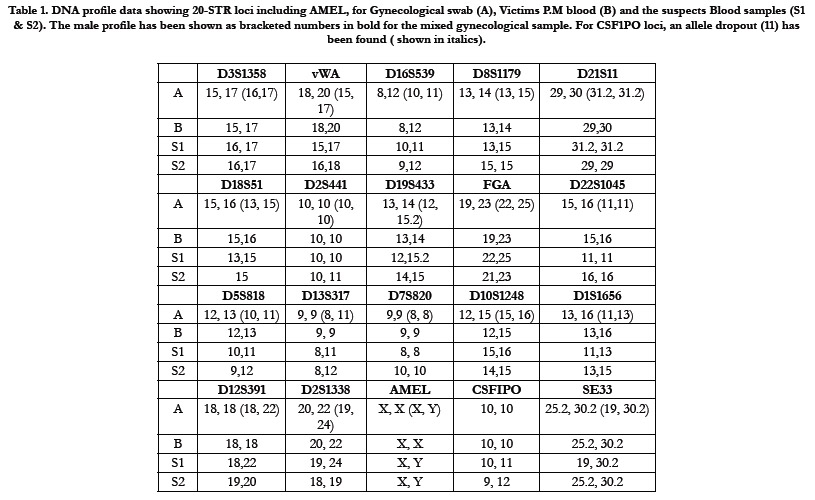
The autosomal profile data for 20 STR including AMEL, for all
the samples (A, B, S1 & S2) has been tabulated (Table 1). The
gynecological swab (A) showed a mixture of two distinct profiles,
which includes the victim (B) and only one of the suspect-1 (S1)
(shown in bold numbers ), thereby excluding S2 form the list of
suspects. However, for CSF-1PO loci, we missed one allele (allele drop out) for the male sample (S1), which may be due to preferential
amplification of the female DNA at the specific locus.
The Amelogenin STR profile-data (column I) for the mixed sample
(A) shown separately in Figure 2., where the ratio of X to Y
peak-area of the mixed sample was calculated considering that
the X/Y allele ratio were balanced in hair sample of the suspect
(S1). The value was found to be 8.08 : 1, which closely resembled
the X/Y ratio ( 7.78 : 1) deducted from the real- time qPCR data
(column II).. The balance of X and Y amplicons is generally used
for predicting the relative contributions of male and female DNA
in mixed samples. However, though, the Amelogenin amplicons
are co-amplified with STR loci in this kit ( Global Filer TM ) the
statistical analysis of the male/female ratio is difficult to perform
as it results in multiple ratios for the multiple autosomal loci. We
have calculated the female to male peak ratios for those alleles in
the mixed profile where the male and female allele components
appeared independently, except D2S1338. The ratio of major/minor
peak heights (except AMEL) were averaged to get one value
for the mixture which was found to be around 2.80 : 1, whereas
for AMEL, alone, the ratio value was calculated to be 3.54 : 1.
This difference might be due to either the omission of the values
for the overlapping male/female peaks in the mixed samples or
due to preferential amplification of male or female template in
some locus. A comprehensive study to resolve the statistical aberration
was not possible in this case owing to the scarcity of the
samples.
To confirm the offender, Y haplo-typing of the gynecological
sample (A) along with the DNA samples of suspects S1 were
conducted using Y-Filer Kit of ThermoFisher and the profiles
have been analyzed. The profiles for 16 Y-Chromosome loci has
been shown in Figure 3. We got a complete match of male profile
with the profile of sample S1, which confirmed the suspect as a
definite perpetrator.
The combination method of autosomal and Y-STR profiling of
the mixed sample is described herein which was quite useful to
solve a sexual assault case where the difference in female to male
DNA in the mixture was relatively less. The method is more time
saving and high throughput as it eliminates the relatively complex
male spermatozoa separation and can easily be used as a routine
lab-work. Therefore, it would be equally effective for any mixed
samples other than semen such as for blood-blood or blood-saliva
combination and samples from azoospermic or vasectomized
male subjects. The application may be extended to resolve the
male-female contributory part in the homicide cases also, where
a blood-blood mixed sample is often traced in the crime scene or
over the victim/accused body.
However, this method has some limitations by itself. As it requires
Y-STR haplo-typing to confirm the perpetrator and as Y-STR
profiles are often not included in national DNA databases, the
acquired Y-STR profile, has to be compared with the Y-STR profile
of known suspects to convict the offender. Since the method
is based on Y-chromosome analysis, female offender cannot be
included in this method. Most importantly, failure to detect any
biological material of the perpetrator from the scene of crime
and/or victim’s sample, not only fails to link an offender to the
crime scene, but it also apparently excludes him as a probable
perpetrator. Therefore, in addition to genotyping, the investigative
agency should take into consideration other circumstantial
evidences including the non-biological exhibits, witness evidence
etc. to include the suspect. Nevertheless, the combination method
helped us to a conclusion in the case described here and pinpoint
the perpetrator.
Even though this study might have some limitations regarding the statistical interpretation of the mixed STR profile, it did not affect
the prime goal to exclude and/or include male subject(s) from the
mixed male/female samples where the difference in contribution
of female to male was relatively less, in the mixed population.
This method is simple, high throughput, which avoids complex
separation of male spermatozoa from the mixed samples and
thereby, may be applicable in day-to-day routine laboratory work.
Figure 1. Electropherogram of the gynecological sample (A), the victim PM blood (B), and the hair samples of the potential suspects (S1 & S2). The profiles are shown for 17 –STR loci including DYS391.
Figure 2. Real-time qPCR data (column I) using QuantFiler Trio kit and STR profile data using GlobalFiler Kit of sex determining marker, AMEL, for the gynecological sample (A) along with the suspect (S1) hair sample (column II) are shown.
Figure 3. Y-chromosome STR analysis data for the gynecological samples (A), and the suspect sample (S1) are shown for 16 loci.
Table 1. DNA profile data showing 20-STR loci including AMEL, for Gynecological swab (A), Victims P.M blood (B) and the suspects Blood samples (S1 & S2). The male profile has been shown as bracketed numbers in bold for the mixed gynecological sample. For CSF1PO loci, an allele dropout (11) has been found ( shown in italics).
Acknowledgement
We acknowledge the support from the Government of West Bengal
and from the Women Safety division, Ministry of Home Affairs
(Government of India) under Nirbhaya Scheme, in terms of
infrastructure development and manpower. We also acknowledge
Sri Sanjoy Mukherjee, Administrator, FSL, West Bengal for all
necessary administrative support.
Compliance With Ethical Standards
All samples used in this study were collected maintaining all legal
formalities and with consent from the persons concerned, wherever
applicable & the method used was as per the guidelines mentioned
in Directorate of Forensic Science of India. This study did
not disclose the identity of anybody by any means.
References
- Benschop CC, Wiebosch DC, Kloosterman AD, Sijen T. Post-coital vaginal sampling with nylon flocked swabs improves DNA typing. Forensic Sci Int Genet. 2010 Feb;4(2):115-21. Pubmed PMID: 20129470.
- Hall A, Ballantyne J. Novel Y-STR typing strategies reveal the genetic profile of the semen donor in extended interval post-coital cervicovaginal samples. Forensic Sci Int. 2003 Sep 9;136(1-3):58-72. Pubmed PMID: 12969621.
- Mayntz-Press KA, Sims LM, Hall A, Ballantyne J. Y-STR profiling in extended interval (> or = 3 days) postcoital cervicovaginal samples. J Forensic Sci. 2008 Mar;53(2):342-8. Pubmed PMID: 18366566.
- Greenspoon SA, Scarpetta MA, Drayton ML, Turek SA. QIAamp spin columns as a method of DNA isolation for forensic casework. J Forensic Sci. 1998 Sep;43(5):1024-30. Pubmed PMID: 9729819.
- Norris JV, Manning K, Linke SJ, Ferrance JP, Landers JP. Expedited, chemically enhanced sperm cell recovery from cotton swabs for rape kit analysis. J Forensic Sci. 2007 Jul;52(4):800-5. Pubmed PMID: 17524064.
- Tsukada K, Asamura H, Ota M, Kobayashi K, Fukushima H. Sperm DNA extraction from mixed stains using the Differex™ system. InInternational Congress Series 2006 Apr;1 (1288): 700-703.
- Gill P, Jeffreys AJ, Werrett DJ. Forensic application of DNA 'fingerprints'. Nature. 1985 Dec 12-18;318(6046):577-9. Pubmed PMID: 3840867.
- Giusti A, Baird M, Pasquale S, Balazs I, Glassberg J. Application of deoxyribonucleic acid (DNA) polymorphisms to the analysis of DNA recovered from sperm. J Forensic Sci. 1986 Apr;31(2):409-17. Pubmed PMID: 3011955.
- Wiegand P, Schürenkamp M, Schütte U. DNA extraction from mixtures of body fluid using mild preferential lysis. Int J Legal Med. 1992;104(6):359- 60. Pubmed PMID: 1515365.
- Adams DE, Presley LA, Baumstark AL, Hensley KW, Hill AL, Anoe KS, et al. Deoxyribonucleic acid (DNA) analysis by restriction fragment length polymorphisms of blood and other body fluid stains subjected to contamination and environmental insults. J Forensic Sci. 1991 Sep; 36(5):1284-98. Pubmed PMID: 1683360.
- Yoshida K, Sekiguchi K, Mizuno N, Kasai K, Sakai I, Sato H, et al. The modified method of two-step differential extraction of sperm and vaginal epithelial cell DNA from vaginal fluid mixed with semen. Forensic Sci Int. 1995 Mar 21;72(1):25-33. Pubmed PMID: 7705732.
- Jankova R, Jakovski Z, Janevski R. Differential extraction method as a golden standard in analyzing semen stains in sexual-assault cases. Forensic Science International: Genetics Supplement Series. 2019 Dec 1;7(1):838-40.
- Maiquilla SM, Salvador JM, Calacal GC, Sagum MS, Dalet MR, Delfin FC, et al. Y-STR DNA analysis of 154 female child sexual assault cases in the Philippines. Int J Legal Med. 2011 Nov;125(6):817-24. Pubmed PMID: 21127891.
- Tobe SS, Dennany L, Vennemann M. An assessment of the subjectivity of sperm scoring. Forensic Sci Int. 2015 Jun;251:83-6. Pubmed PMID: 25863702.
- Vuichard S, Borer U, Bottinelli M, Cossu C, Malik N, Meier V, et al. Differential DNA extraction of challenging simulated sexual-assault samples: a Swiss collaborative study. Investig Genet. 2011 May 4;2:11. Pubmed PMID: 21542912.
- Lounsbury JA, Nambiar SM, Karlsson A, Cunniffe H, Norris JV, Ferrance JP, et al. Enhanced recovery of spermatozoa and comprehensive lysis of epithelial cells from sexual assault samples having a low cell counts or aged up to one year. Forensic Sci Int Genet. 2014 Jan;8(1):84-9. Pubmed PMID: 24315594.
- Di Martino D, Giuffrč G, Staiti N, Simone A, Sippelli G, Tuccari G, et al. LMD as a forensic tool in a sexual assault casework: LCN DNA typing to identify the responsible. InInternational Congress Series 2006 Apr;1(1288): 571-573.
- Murray C, McAlister C, Elliott K. Identification and isolation of male cells using fluorescence in situ hybridisation and laser microdissection, for use in the investigation of sexual assault. Forensic Sci Int Genet. 2007 Dec;1(3- 4):247-52. Pubmed PMID: 19083769.
- Sanders CT, Sanchez N, Ballantyne J, Peterson DA. Laser microdissection separation of pure spermatozoa from epithelial cells for short tandem repeat analysis. J Forensic Sci. 2006 Jul;51(4):748-57. Pubmed PMID: 16882215.
- Vandewoestyne M, Van Hoofstat D, Van Nieuwerburgh F, Deforce D. Automatic detection of spermatozoa for laser capture microdissection. Int J Legal Med. 2009 Mar;123(2):169-75. Pubmed PMID: 18661142.
- Gill P, Lygo JE, Fowler SJ, Werrett DJ. An evaluation of DNA fingerprinting for forensic purposes. Electrophoresis. 1987;8(1):38-44.
- Hulme P, Lewis J, Davidson G. Sperm elution: an improved two phase recovery method for sexual assault samples. Sci Justice. 2013 Mar;53(1):28-33. Pubmed PMID: 23380059.
- Chen J, Kobilinsky L, Wolosin D, Shaler R, Baum H. A physical method for separating spermatozoa from epithelial cells in sexual assault evidence. J Forensic Sci. 1998 Jan;43(1):114-8. Pubmed PMID: 9456531.
- Garvin AM. Filtration based DNA preparation for sexual assault cases. J Forensic Sci. 2003 Sep;48(5):1084-7. Pubmed PMID: 14535671.
- Horsman KM, Barker SL, Ferrance JP, Forrest KA, Koen KA, Landers JP. Separation of sperm and epithelial cells in a microfabricated device: potential application to forensic analysis of sexual assault evidence. Anal Chem. 2005 Feb 1;77(3):742-9. Pubmed PMID: 15679339.
- Norris JV, Evander M, Horsman-Hall KM, Nilsson J, Laurell T, Landers JP. Acoustic differential extraction for forensic analysis of sexual assault evidence. Anal Chem. 2009 Aug 1;81(15):6089-95. Pubmed PMID: 19591449.
- Liu W, Chen W, Liu R, Ou Y, Liu H, Xie L, Lu Y, Li C, Li B, Cheng J. Separation of sperm and epithelial cells based on the hydrodynamic effect for forensic analysis. Biomicrofluidics. 2015 Aug 31;9(4):044127. Pubmed PMID: 26392829.
- Schoell WM, Klintschar M, Mirhashemi R, Pertl B. Separation of sperm and vaginal cells with flow cytometry for DNA typing after sexual assault. Obstet Gynecol. 1999 Oct;94(4):623-7. Pubmed PMID: 10511370.
- Hudlow WR, Buoncristiani MR. Development of a rapid, 96-well alkaline based differential DNA extraction method for sexual assault evidence. Forensic Sci Int Genet. 2012 Jan;6(1):1-16. Pubmed PMID: 21288791.
- Li XB, Wang QS, Feng Y, Ning SH, Miao YY, Wang YQ, et al. Magnetic bead-based separation of sperm from buccal epithelial cells using a monoclonal antibody against MOSPD3. Int J Legal Med. 2014 Nov;128(6):905-11. Pubmed PMID: 24590379.
- Zhao XC, Wang L, Sun J, Jiang BW, Zhang EL, Ye J. Isolating Sperm from Cell Mixtures Using Magnetic Beads Coupled with an Anti-PH-20 Antibody for Forensic DNA Analysis. PLoS One. 2016 Jul 21;11(7):e0159401. Pubmed PMID: 27442128; PMCID: PMC4956189.
- Anslinger K, Bayer B, Danilov SM, Metzger R. Application of sperm-specific antibodies for the separation of sperm from cell mixtures. Forensic Science International: Genetics Supplement Series. 2008 Aug 1;1(1):394-5.
- Wang Q, Ning S, Li X. Isolation of sperm cells from mixed stains by immunomagnetic bead. Chin J Forensic Med. 2013; 28:317–319.
- Voorhees JC, Ferrance JP, Landers JP. Enhanced elution of sperm from cotton swabs via enzymatic digestion for rape kit analysis. J Forensic Sci. 2006 May;51(3):574-9. Pubmed PMID: 16696704.
- Roewer L. Y chromosome STR typing in crime casework. Forensic Sci Med Pathol. 2009;5(2):77-84. Pubmed PMID: 19455440.
- Martin P AC. Application of Y-STR analysis to rapes that cannot be solved by autosomal STR. Progress in Forensic Genet. 2000;526?528.
- Kumar N, Maitray A, Gupta R. Importance of Y-STR profiling in sexual assault cases with mixed DNA profile. Int J Mol Biol Open Access. 2018;3(1):42-5.
- Prinz M, Ishii A, Coleman A, Baum HJ, Shaler RC. Validation and casework application of a Y chromosome specific STR multiplex. Forensic Sci Int. 2001 Sep 1;120(3):177-88. Pubmed PMID: 11473800.
- Prinz M, Sansone M. Y chromosome-specific short tandem repeats in forensic casework. Croat Med J. 2001 Jun;42(3):288-91. Pubmed PMID: 11387641.
- Hall A, Ballantyne J. The development of an 18-locus Y-STR system for forensic casework. Anal Bioanal Chem. 2003 Aug;376(8):1234-46. Pubmed PMID: 12830356.
- Cerri N, Ricci U, Sani I, Verzeletti A, De Ferrari F. Mixed stains from sexual assault cases: autosomal or Y-chromosome short tandem repeats? Croat Med J. 2003 Jun;44(3):289-92. Pubmed PMID: 12808720.
- Kayser M, Cagliŕ A, Corach D, Fretwell N, Gehrig C, Graziosi G, et al. Evaluation of Y-chromosomal STRs: a multicenter study. Int J Legal Med. 1997;110(3):125-33. Pubmed PMID: 9228563.
- Jobling MA, Tyler-Smith C. The human Y chromosome: an evolutionary marker comes of age. Nat Rev Genet. 2003 Aug;4(8):598-612. Pubmed PMID: 12897772.
- Gill P, Brenner C, Brinkmann B, Budowle B, Carracedo A, Jobling MA, et al. DNA Commission of the International Society of Forensic Genetics: recommendations on forensic analysis using Y-chromosome STRs. Forensic Sci Int. 2001 Dec 15;124(1):5-10. Pubmed PMID: 11741752.
- Thangaraj K, Reddy AG, Singh L. Is the amelogenin gene reliable for gender identification in forensic casework and prenatal diagnosis? Int J Legal Med. 2002 Apr;116(2):121-3. Pubmed PMID: 12056520.
- Butler JM. Forensic DNA typing: biology, technology, and genetics of STR markers. Elsevier; 2005 Feb 8.
- Vermeulen M, Wollstein A, van der Gaag K, Lao O, Xue Y, Wang Q, Roewer L, Knoblauch H, Tyler-Smith C, de Knijff P, Kayser M. Improving global and regional resolution of male lineage differentiation by simple single-copy Y-chromosomal short tandem repeat polymorphisms. Forensic Sci Int Genet. 2009 Sep;3(4):205-13. Pubmed PMID: 19647704.
- Purps J, Geppert M, Nagy M, Roewer L. Validation of a combined autosomal/Y-chromosomal STR approach for analyzing typical biological stains in sexual-assault cases. Forensic Sci Int Genet. 2015 Nov;19:238-242. Pubmed PMID: 26280567.
- Clayton TM, Whitaker JP, Sparkes R, Gill P. Analysis and interpretation of mixed forensic stains using DNA STR profiling. Forensic Sci Int. 1998 Jan 9;91(1):55-70. Pubmed PMID: 9493345.
- Gill P, Sparkes R, Pinchin R, Clayton T, Whitaker J, Buckleton J. Interpreting simple STR mixtures using allele peak areas. Forensic Sci Int. 1998 Jan 9;91(1):41-53. Pubmed PMID: 9493344.
- Alonso A, Martín P. A real-time PCR protocol to determine the number of amelogenin (X-Y) gene copies from forensic DNA samples. Methods Mol Biol. 2005;297:31-44. Pubmed PMID: 15570098.
- Allen RW, Fuller VM. Quantitation of human genomic DNA through amplification of the amelogenin locus. J Forensic Sci. 2006 Jan;51(1):76-81. Pubmed PMID: 16423226.
- Kind SS. The use of the acid phosphatase test in searching for seminal stains. J. Crim. L. Criminology & Police Sci. 1957; 47:597–600.
- Kind SS. The acid phosphatase test. In: Curry A, editor. Methods of forensic science. London: Interscience; 1964; 267–288.
- Hochmeister MN, Budowle B, Rudin O, Gehrig C, Borer U, Thali M, et al. Evaluation of prostate-specific antigen (PSA) membrane test assays for the forensic identification of seminal fluid. J Forensic Sci. 1999 Sep;44(5):1057- 60. Pubmed PMID: 10486959.
- Liu JY, Zhong C, Holt A, Lagace R, Harrold M, Dixon AB, Brevnov MG, Shewale JG, Hennessy LK. AutoMate Express™ forensic DNA extraction system for the extraction of genomic DNA from biological samples. J Forensic Sci. 2012 Jul;57(4):1022-30. Pubmed PMID: 22390771.
- Davis CP, King JL, Budowle B, Eisenberg AJ, Turnbough MA. Extraction platform evaluations: a comparison of AutoMate Express™, EZ1® Advanced XL, and Maxwell® 16 Bench-top DNA extraction systems. Leg Med (Tokyo). 2012 Jan;14(1):36-9. Pubmed PMID: 22182593.
- Livak KJ. Allelic discrimination using fluorogenic probes and the 5' nuclease assay. Genet Anal. 1999 Feb;14(5-6):143-9. Pubmed PMID: 10084106.