Physical, Proximate and Toxicological Properties of Andrographis Paniculata Herbal Powder Beverage Mix
Etti CJ1*, Etti I C2, Sani D3
1 Department of Agricultural and Food Engineering, University of Uyo, Uyo, Nigeria.
2 Department of Pharmacology and Toxicology, University ofUyo, Uyo, Nigeria.
3 Department of Veterinary Pharmacology and Toxicology, Faculty of Veterinary Medicine, Ahmadu Bello University, Zaria, Nigeria.
*Corresponding Author
Etti CJ,
Department of Agricultural and Food Engineering,
University of Uyo, Uyo 520271, Nigeria.
E-mail: christopheretti@uniuyo.edu.ng
Received: April 17, 2020; Accepted: May 18, 2020; Published: May 22, 2020
Citation: Etti CJ, Etti I C, Sani D. Physical, Proximate and Toxicological Properties of Andrographis Paniculata Herbal Powder Beverage Mix. Int J Food Sci Nutr Diet. 2020;9(2):448-454. doi: dx.doi.org/10.19070/2326-3350-2000080
Copyright: Etti CJ© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
This study investigated the physical, proximate and toxicological properties of Andrographis paniculata (A. paniculata) herbal powder beverage mix. The herbal powder, A. paniculata with high medicinal value was formulated with non-dairy creamer and sugarto enhance the taste, nutritional and economic values, thus making itgood and safe for human use. The physical properties of the formulated herbal beverage mix was determined: Particle size, pH, moisture content, bulk and tapped densities, were measured to determine Carr index and Hausner ratio which were indices of flowability. The proximate analysis such as ash, lipid, fibre, carbohydrate and crude protein contents of the beverage mix was determined. Toxicological study on the formulated beverage mix was performed using healthy male wistar rats to ascertain the safety of the product. The formulation of the pure A. paniculata herbal powder into a beverage mix enhanced the particle sizes with reduction in moisture content making the beverage to be “free flowing”. The formulated A. paniculata beverage mix had a pH of 7.49 ± 0.11 which is suitable for the human system. The formulated beverage mix was a good source of carbohydrate (87.81 ± 0.30%) and protein (8.00 ± 0.24%). However, the data revealed that the formulation was not a rich source of fat, making it recommendable to people with high lipid profile. The safety of the mix was ascertained after the acute oral and sub-chronic toxicity studies carried out for 28 days revealed no apparent adverse effect on the histology ofboth the liver and kidney of experimental animals.
2.Introduction
3.Materials and Methodology
4.Results and Discussions
5.Conclusion
6.Acknowledgement
7.References
Keywords
Physical Properties; Proximate Analysis; Toxicological Properties; Andrographis Paniculata; Herbal Powder; Beverage Mix.
Introduction
Herbal products, which are increasing worldwide are often classified as dietary supplements, but are not subject to premarketing approval unlike pharmaceutical products [1]. Companies and individuals who market these products have the sole responsibility to ensure the safety of their products. Most often, manufacturers produce, sell, and market herbs without first demonstrating safety and efficacy [2]. The setback in the production of herbal productsand medicine is standardization, a process involving the production of herbal resources like extracts or phytochemicals with guaranteed products potency and stability in active compound content level [3]. Thus, high level of knowledge and skills in phytochemical analysis and process technology are necessary to ensure the required quality assurance. There is a large global market for herbal products because of their nutritive and medicinal properties [1, 4]. In fact, an estimated 70,000 species of plants are used for medicinal purposes globally [5, 6]. Finished herbal products are presented in various dosage forms like decoctions, herbal powders, alcoholic beverages, capsules, tablets, ointments and creams [7]. Generally, the therapeutic usage of herbs is enhanced in powder forms owing to increase absorption due to the large surface area [8]. The bulk behaviour of herbal powders are influenced by their physical properties such as particle sizes and shapes distribution, weights, chemical composition as well as their moisture contents [9]. Herbal powders with very fine particle size ranges may give rise to problems in the course of their industrial processing [10]. Ethno-botanically, as reported by Ayodele et al., [11], herbs and spices have been significantly utilised traditionally due to their flavour enhancement properties and their medicinal values [1].
Andrographis Paniculata (A. paniculata) also known as “king of bitters”, is a herbal plant that is widely used in traditional medicines for centuries in the treatment of certain diseases like skin eruptions, boils, scabies, and chronic undetermined fevers. A. paniculata has been reported to possess antibacterial, antifungal, antiviral, hypoglycemic, anti-cancer, anti- inflammatory and antipyretic properties [12]. The major modern use of A. paniculata is for the prevention and treatment of cold as it possesses antithrombotic actions, suggesting a likely advantage in cardiovascular disease [13].
The bitter taste of A. paniculata may influence its usage, thus, necessitating its formulation with creamer and sugar into a beverage mix in order to enhance its acceptability while ensuring the safety. A key stage in ensuring the safety of a product is to conduct toxicity tests in appropriate animal models [14]. Acute toxicity studies and repeated dose toxicity tests were employed to predict the safety in humans. The aim of this research was to investigate the Physical, Proximate and toxicological properties of A. paniculata herbal powder mix beverage.
Materials and Methodology
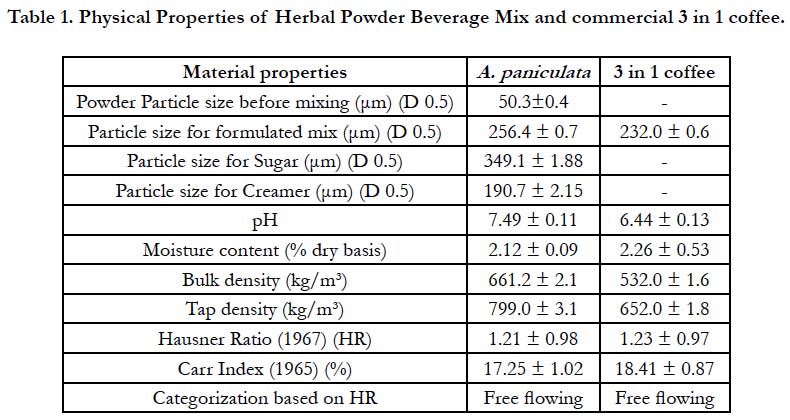
The herbal powder, A. paniculata, used in this study was acquired from Ethno Resources Sdn. Bhd., Sungai Buloh, Malaysia. The powder was produced after the herb was oven dried and ground to smaller standard particle sizes to obtain the powder. The material properties used in this work are summarized in Table (1). Sugar (MSM Perlis Sdn. Bhd. brand) and the nondairy creamer (Carnation brand) were bought from KK supermarket in Malaysia with particle size (D0.5) of 349.1 ± 1.88μm and 190.7 ± 2.15μm respectively. A. paniculata powder of particle sizes (less than 100μm), was formulated into herbal powder mix by mixing it with other materials like sugar and creamer in a ratio of 1:10:10 respectively using a tumbler mixer (Glas-col, TERRE HAUTE, USA) at 30 rpm for 30 minutes per each formulation to ensure consistency and homogeneity of the herbal powder beverage mix. The ratio of “1” for the A. paniculata herbal powder was chosen to ensure the safety of the herbs [15]. The ratio “10” for sugar was chosen to enhance the sweet taste of the mix and also for nutrients enhancement (Carbohydrates) [16], and the ratio “10” for the nondairy creamer was chosen for nutrients enhancement as well.A commercial “3 in 1” Ipoh white coffee powder bought from KK supermarket was used to compare with the results of the formulated herbal powder mix in this study.
The pH of the materials was checked using the pH meter (mV/ORP, METTLER TOLEDO, USA) in the Department of Process and Food Engineering Laboratory, UPM. 5 grams of powder was weighed and transferred into a measuring flask, 100 ml of distilled water was added into the flask and shaking was done for a minute. The solution was allowed to settle for one hour. The pH meter was calibrated and an appropriate amount of the clear aqueous solution was transferred from the flask into the beaker. The pH value was then measured and reported.
The moisture content of A. paniculata beverage mix powder was determined according to AOAC [17], with the use of a digital moisture analyzer (OHAUS MB45, UK). The A. paniculata beverage mix powder (1.0 ± 0.01 g) was weighed and kept in a sample plate,then measurement was made in triplicate. The result is given in Table 1.
Bulk density (ρbulk) can be identified as the volume occupied by the solid plus the volume of voids when divided into powder. Whereas, tap density (ρtab) is a different type of bulk density obtained by tapping or vibrating the container in a particular method to achieve more effective particle parking and therefore, it is usually higher than bulk density [18],
ρbulk = Wt / Vbulk ----- (1)
Where Wt is weight of the powder, Vbulk is volume of the powder obtained from tarred graduated cylinder without tapping. The tapped density (ρtab) of the powders was calculated by using the following equation;
ρtab = Wt / Vtab ----- (2)
Where Wt is the weight of powder, Vtab is the volume of the powder bed after 500 taps.
Carr Index and Hausner Ratio are used in describing the flowability of powder. Carr Index (CI.) can be determined as the ratio of the difference of the tapped and the bulk densities to the tapped density [19]. According to Carr [19], who introduced the flowability index, an excellent flowability is between the Carr Index of 5% to 15% while Carr Index of above 25% normally shows poor flowability.
C.I = ρtap - ρbulk / ρtap ---- (3)
Hausner Ratio (HR) are also used to characterize the flowability of powder, which can be determined by the ratio of the tapped density to that of bulk density [20]. HR of 1.0 to 1.1, indicates free flowing powder, HR greater than 1.1 to 1.25, indicates powder that is medium flowing, HR greater than 1.25 to 1.4, indicates powder that is difficult to flow and HR higher than 1.4, indicates powder that is very difficult to flow [21];
H.R = ρtap / ρbulk ----- (4)
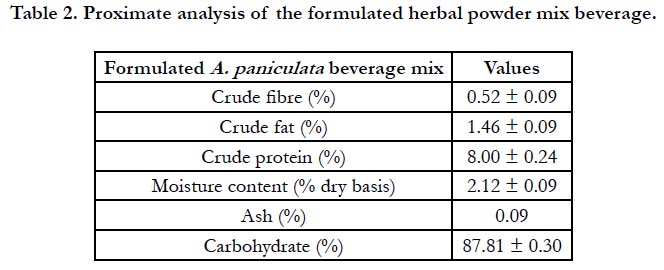
Proximate composition of the samples was carried out using official AOAC [22] methods for moisture content, crude fat, crude fibre, ash, crude protein. Nitrogen to protein conversion factor of 6.25 was used. Carbohydrate content was calculated by difference. The result of the proximate analysis of the formulated herbal powder mix beverage is shown in Table 2.
Healthy, mature male Wistar rats, weighing between 150-170g and aged 10-12 weeks were used for this experiment. The animals were procured from Saintik Enterprise, No 5, Jalan 1/4 Taman Kembangsari, 43300, Seri kembangan, Selangor, Malaysia. The rats were kept in stainless steel cages for a period of 10 days to adapt to laboratory condition at an ambient temperature of 25 ± 2°C with 12h light: 12 h dark cycle. They were fed on commercial rat/mouse pellet diet (Specialty feeds, Glen forest, Western Australia) and water ad libitum throughout the study duration. All animal experiments were conducted in compliance with the internationally accepted principles of laboratory animal care specified and approved by UPM Animal Care Use Committee (UPM/IACUC/AUP-R061/2014).
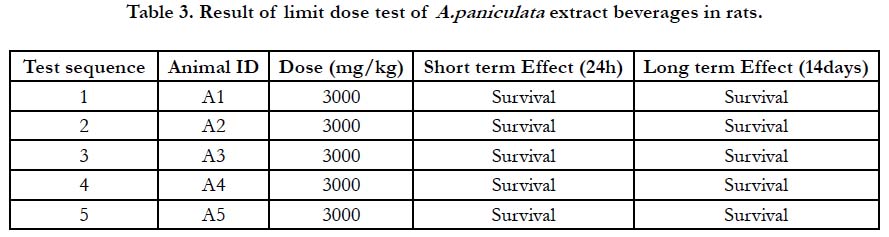
The limit dose test (LD50), up and down procedure [23] originally outlined by the Organization for Economic Co-operation and Development [24] was adopted with slight modification to evaluate the acute oral toxicity of A. paniculata extract mixed beverages following oral administration in adult male rats. A total of five (5) male rats were randomly selected and used for the study. The rats were maintained on standard feed and water provided ad libitum. They were marked and caged individually in the laboratory for 7 days to allow for acclimatization to the laboratory conditions. The rats to be treated were fasted overnight but allowed free access to water prior to dosing on each occasion. Each of the ratswas picked, weighed and dosed orally with a single high dose of 3000mg/kg body weight of the extract beverages suspended in water (vehicle). Similar treatment was done for another set of rats with distilled water and was assigned control group. All the animals were observed thoroughly for instant death and then watched for the successive 24 hours for the short-term outcome and finally for the next 14 days for any delayed toxic effects. The LD50 was predicted if three or more rats survived after the experimental period [23].
Male Wistar rats were randomly selected and used for the experiment. The rats were assigned into four groups (n=6 per group). Groups C, D, and E were administered graded doses (50, 100 and 200mg/kg respectively) of the extract beverages suspended in water orally for 28 days, while group A (the control) and B (positive control) received distilled water and 200mg of the commercial preparation for the same period. The weight of the rats were measured prior to administration of the extract and then weekly thereafter. Similarly, toxic manifestations and mortality were monitored daily for 28 days.
At the end of the experimental period (29th day), blood samples were collected via cardiac puncture from the rats in each group, transferred into plain and EDTA containing tubes for biochemical and haematological analysis respectively prior to sacrifice. The liver, kidney, heart, lung, intestine, stomach and pancreas of the sacrificed animals were also removed, physically examined for possible lesions and samples collected for histopathological analysis using standard Methods [25].
Estimation of haematological parameters such as red blood cells count (RBC), white blood cells count (WBC), haemoglobin concentration (Hb) and platelet (PLT) were carried out using a fully automated haematological analyser Abbott Cell-Dyn 3500 (Abbott Laboratories, IL, USA), while serum biochemistry tests were performed using a COBAS Integra 800 (Roche, Germany).
Samples of liver, kidney, and spleen isolated from individual rats were fixed in 10% buffered formalin. The tissues were then dehydrated through graded concentrations of ethanol (70%, 95% and absolute), cleared in xylene and embedded in paraffin wax. The embedded tissues were sectioned at 5μm thickness and stained with Haematoxylin and Eosin (H&E) for light microscope examination [25-27]. The lesions observed under microscope were captured for further analysis.
Table 1 below shows the physical Properties of herbal powder beverage mixand the commercial 3 in 1 coffee. From the table, the particle size analysis (D0.5) of pure herbal powder, sugar, creamer and the formulated herbal powder beverage mix product is shown. The formulation of the pure A. paniculata herbal powder into a beverage mix enhanced the particle sizes from 50.3 ± 0.4μm of A. paniculata pure powder to 256.4 ± 0.7μm of A. paniculata beverage mix. It was observed that based on Carr index [19] and Hausner ratio [20], the formulated beverage mix was “Free flowing” in terms of flowability properties. The enhancement in particle size enhanced the flowability because the smaller the particle size, the more difficult it is for the particle to flow [28]. From Table 1, a commercial 3 in 1 coffee mix was also analyzed and the result showed that it was also “Free flowing”. The free flowing nature of the formulated beverage will reduce the cost of controlling caking problem in the industry. Also, considering the pH analysis, the formulated A. paniculata was seen to have a pH of 7.49 ± 0.11 which was neutral to basic in nature compared to the commercial 3 in 1 coffee mix with pH of 6.44 ± 0.13 which was acidic in nature. Alkaline foods help in countering the risks of acidity and acid refluxes, bringing some sort of relief to the body system [29].
Proximate analysis plays important role in assessing the nutritional significance of food [30]. The world health organization (WHO) had emphasized the need and importance of determining the proximate analysis of herbal formulations for standardization purposes [31]. The proximate analysis of A. paniculata herbal powder beverage formulation is shown in Table 2 below. A. paniculata herbal powder beverage mix was high in the crude protein content (8.00 ± 0.24%). Generally, the data revealed that the formulation was not a rich source of fats and can therefore be recommended to people that fat is a risk factor. Also, the low fat contents is a reason why all the herbal powders beverage mix isfree flowing (see Table 1) because fat content can influence the flowability of powders, high fat contents will make the powders to be cohesive, posing a problem to flow [32]. A. paniculata herbal powder beverage mix is recommended for people that have protein deficiency because of its rich protein content. The herbal powder beverage mix could also serve as an energy buster due to its high carbohydrate content (87.81 ± 0.30%) (Table 2). The low moisture content of the herbal powder beverage mix (2.12 ± 0.09%) is also responsible for its free flowing nature (Table 1) because high moisture contents increase powders cohesive strength [33].
In this study, administration of 3000mg/kg of the extract beverages did not cause any visible behavioural change and death of the rats within the short and long outcome of the limit dose test (Table 3). All five rats survived until the end of the experiment with no mortality recorded. The LD50 was determined to be greater than 3000mg/kg body weight [23].
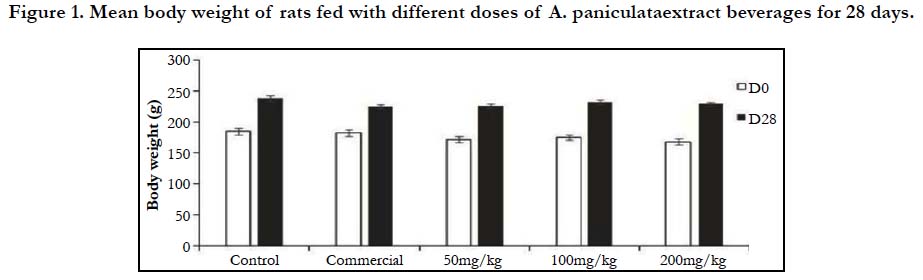
Figure 1 depicts changes in body weight of rats fed with different doses (50, 100 and 200 mg/kg) of A. paniculata extract beverage mix for 28 days. There was significant (P <0.05) dose dependent increase in the mean body weight in both the control and treated groups compared to respective day 0 values.
Figure 1. Mean body weight of rats fed with different doses of A. paniculataextract beverages for 28 days.
In subchronic toxicity study,there were no observable changes in the general behaviourof all the treated rats (groups 50mg/kg, 100mg/kg and 150mg/kg) as compared to thecontrol groups (Control andCommercial control). Both controls and the treated rats appeared uniformly healthy till the end of the study and throughout the 28 days treatment period.
Analyses: Exposure of rats to sub chronic treatment with varied doses (50, 100 and 200mg/kg) for 28 days showed significant (P <0.05) increasein the haematological parameters(red blood cell count (RBC), haemoglobin concentration (HGB), total white blood cellcount (WBC), white blood cell differential count and platelet counts of the extract formulated treated rats when compared to control rats. Exposure of rats to sub chronic treatment with varied doses (50, 100 and 200mg/kg) for 28 days showed significant (P <0.05) increase in the haematological parameters (red blood cell count (RBC), haemoglobin concentration (HGB), and platelet counts (PLT) of the extract formulated treated rats when compared to control rats. With the increasing questioning of the safety of herbal (medicinal plant) usage sequel to frequent report of illness and fatalities, research into evaluating the safety profile on medicinal plant becomes necessary. Haematological analysis of blood parameters in animal study is necessary to evaluate the risk of alterations of the hematopoietic system in toxicity studies, for necessary application to humans [34]. Haematopoiesis is the process of blood cell formation including an erythrocyte (RBC), a leukocyte (WBC), or a thrombocyte (platelet). In healthy adults, stem cells in hematopoietic sites undergo a series of divisions and maturational changes to give rise to mature cells found in the blood [35]. In this study, administration of the herbal powder formulated beverage mix after 28 days up to a dose of 200mg/kg body weight resulted in dose-dependent significant increase in RBC, HGB and PLT in A. paniculata formulated beverage mix (Table 4). This could be as a result of the activation of the hematopoietic system, leading to the production of RBC (erythropoiesis) and platelets [21]; although, all the haematological parameters (WBC, RBC, HGB and PLT) were within rat hematologic reference ranges [36]. There was a slight insignificant increase in WBC for the formulated beverages mix compared with the control. The WBC protects the body from infection by foreign organisms, the RBC boost the immune system and the platelets protect blood vessels from endothelial damage as well as initiate repair of these vessels. This therefore suggests a strong immuno-modulatory, antioxidant and endothelial protection and repair activity of these formulated beverage mix.
Table 4. Haematological Values of Rats Treated with A. paniculata Herbal Powder Beverage Mix for 28 Days.
Biochemical analysis did not reveal any significant difference after 28 days of exposure of rats to different doses of A. paniculata extract formulation when compared to control group. However, there were increases in the levels of AST, ALT and ALP in the group treated with 200mg/kg of the extract formulation when compared to control although not significant. Tables 5 showed the biochemical analysis of the different formulated herbal powder beverage mix. Biochemical analysis did not reveal any significant difference after 28 days of exposure of rats to different doses of all formulated beverages mix when compared to control group. Liver is the major organ involved in drug biotransformation. Levels of serum liver biomarker enzymes are biochemical parameters usually performed in order to evaluate any toxic effects on the liver [37]. Increases in the levels of AST, ALT and ALP in the serum are associated with liver toxicity by drugs or any other hepatotoxin [38]. More so, ALT is more specific to liver and thus a better parameter for detecting liver injury as ALP is present mostly in cells lining the billiary duct of the liver and is used to diagnose obstruction to the billiary system. Therefore, its elevation in the blood indicates cholestatic diseases such as gallstone or tumor blocking the bile duct [39]. Urea and creatinine are considered as important markers of kidney dysfunction [40]. In this study, chronic exposure of rats to different doses (50, 100 and 200mg/kg) of herbal powder formulated beverage mix did not revealed significant changes in all the biochemical parameters tested over the 28 days treatment compared to control group, signifying normal functioning of the liver and kidney. It also confirms with the theory of target organ toxicity [41], since the kidney is the major organ of excretion, suggesting that the toxic principles in these beverages mix are not high enough to induce significant health hazard [25].
Table 5. Biochemical Parameters of Rats Treated with A. paniculata Herbal Powder Beverage Mix for 28 Days.
Histological evaluations of the livers and kidneys did not show any morphological changes after administration of the aqueous extracts formulation for 28 days. The herbal powder formulated beverages mixes are relatively safe as they reveal no apparent adverse side effect on the function of both the liver and kidney (see Figures 2 and 3). However care should be taken with prolonged consumption of higher doses (>200mg/kg) of this herbal powder formulated beverages.
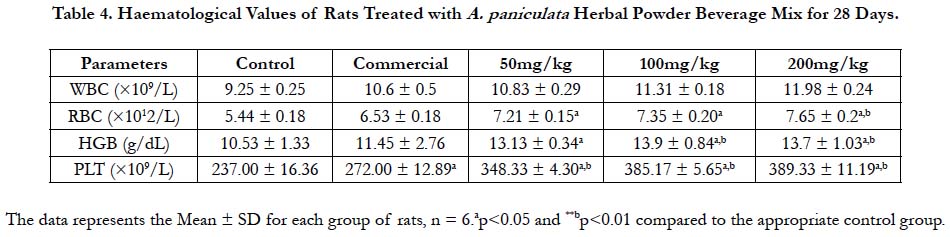
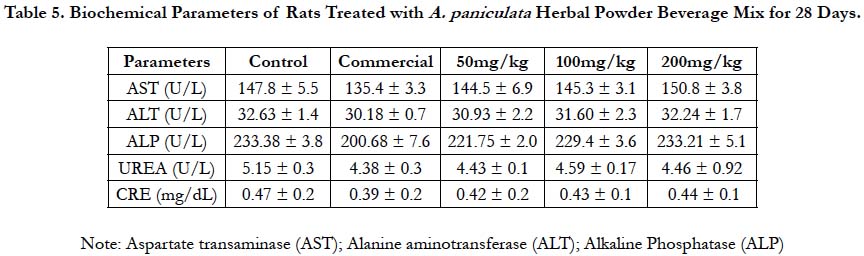
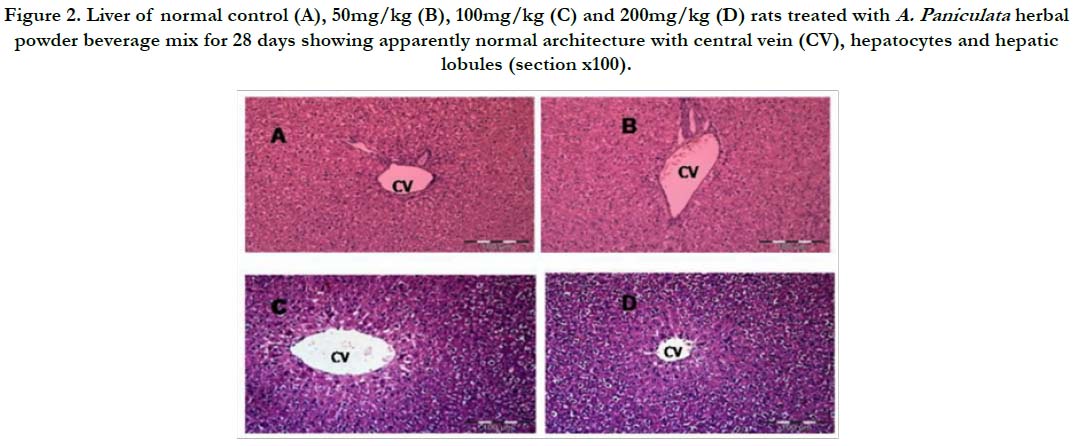
Figure 2. Liver of normal control (A), 50mg/kg (B), 100mg/kg (C) and 200mg/kg (D) rats treated with A. Paniculata herbal powder beverage mix for 28 days showing apparently normal architecture with central vein (CV), hepatocytes and hepatic lobules (section x100).
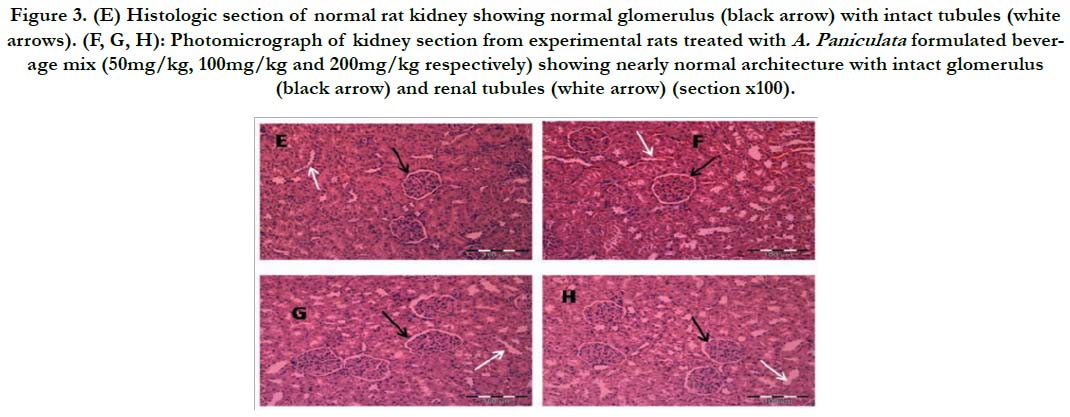
Figure 3. (E) Histologic section of normal rat kidney showing normal glomerulus (black arrow) with intact tubules (white arrows). (F, G, H): Photomicrograph of kidney section from experimental rats treated with A. Paniculata formulated beverage mix (50mg/kg, 100mg/kg and 200mg/kg respectively) showing nearly normal architecture with intact glomerulus (black arrow) and renal tubules (white arrow) (section x100).
Conclusion
In conclusion, this study enhances the understanding of powder physical property, which can improve the development and formulation of herbal powder beverage mix. The formulated beverage mix could serve as an energy buster and body building beverage because it is a good source of carbohydrate and protein. The extract formulation is relatively safe asthe administration of 3000mg/kg of the aqueous beverage did not elicit any sign of toxicity or cause any visible behavioural change and death of the animals within the short and long outcome of the limit dose test. Analysis of the haematological, biochemical and histological parameters of the rats after the administration of the aqueous beverage formulation for 28 days revealed the safety of the beverage mix. However care should be taken with prolonged consumption of higher doses (> 200mg/kg) of this extract formulation.
Acknowledgement
Authors are grateful to technologies at the Department of Process and Food Engineering Laboratory and staff at animal house Universiti Putra Malaysia (UPM) Malaysia, where the work was carried out.
References
- Alexandra N Welz, Agnes Emberger-Klein, Klaus Menrad. Why people use herbal medicine: insights from a focus-group study in Germany. BMC Complementary and Alternative Medicine. 2018; 92(18):2160-6. PMID: 29544493.
- Stephen Bent. Herbal medicine in the United States: review of efficacy, safety, and regulation: grand rounds at University of California, San Francisco Medical Center. Journal of General Internal Medicine. 2008; 23(6): 854–859. PMID: 18415652.
- Kunle OF, Egharevba HO, Ahmadu PO. Standardization of herbal medicines- A review. International Journal of Biodiversity and Conservation. 2012 Mar 20;4(3):101-112.
- Issa RA, Basheti IA. Herbal products use among chronic patients and its impact on treatments safety and efficacy: a clinical survey in the Jordanian field. Trends Med. Res. 2016;12:32-44.
- Chatfield K, Salehi B, Javad SJ, Afshar L. Applying an Ethical Framework to Herbal Medicine. Evidence-Based Complementary and Alternative Medicine. 2018. PMID: 30327677.
- Shilin Chen, Xiaohui Pang, Jingyuan Song, Linchun Shi, Hui Yao, Jianping Han, et al. A renaissance in herbal medicine identification: From morphology to DNA. Biotechnology Advances. 2014; 32(7): 1237–1244. PMID: 25087935.
- Ozioma EO, Chinwe OA. Herbal medicines in African traditional medicine. Herbal Medicine. 2019 Jan 30;10:191-214.
- S MalekiDizaj, ZhVazifehasl, S Salatin, KhAdibkia, Y Javadzadeh. Nanosizing of drugs: Effect on dissolution rate. Research in pharmaceutical science. 2015; 10(2): 95–108. PMID: 26487886.
- Fu X, Huck D, Makein L, Armstrong B, Willen U, Freeman T. Effect of particle shape and size on flow properties of lactose powders. Particuology. 2012 Apr 1;10(2):203-8.
- Shanmugam S. Granulation techniques and technologies: recent progresses. Bioimpacts. 2015; 5(1): 55-63. PMID: 25901297.
- Asowata-Ayodele AM, Afolayan AJ, Otunola GA. Ethnobotanical survey of culinary herbs and spices used in the traditional medicinal system of Nkonkobe Municipality, Eastern Cape, South Africa. South African journal of botany. 2016 May 1;104:69-75.
- Singh A, Khan MM, Sahu D, Vishwakarma N, Yadav A, Singh AN. Pharmacological and anti-bacterial activities of the leaves of AndrographispaniculataNees. Journal of Pharmacognosy and Phytochemistry. 2017;6(3):418-20.
- Amroyan E, Gabrielian E, Panossian A, Wikman G, Wagner H. Inhibitory effect of andrographolide from Andrographispaniculata on PAF-induced platelet aggregation. Phytomedicine. 1999 Mar 1;6(1):27-31.
- Arome D, Chinedu E. The importance of toxicity testing. Journal of Pharmaceutical and BioSciences. 2013 Dec; 4:146-8.
- Gamaniel KS. Toxicity from medicinal plants and their products. Nigerian Journal of Natural Products and Medicine. 2000;4(1):4-8.
- Hinkova A, Bubnik Z, Kadlec P. Chemical composition of sugar and confectionery products. Handbook of Food Chemistry. 2015:585-626.
- AOAC. Official methods of analysis. Association of Official Analytical Chemists. 18th Ed. Arlington, VA. 2005; 806-842.
- Lowell S, Shields JE. Density measurement. In Powder Surface Area and Porosity .Springer, Dordrecht.1984;pp-217-221.
- Carr RL. Evaluating flow properties of solids. Chemical Engineering.1965; 72: 163-168.
- Hausner HH.Friction conditions in a mass of metal powder. International Journal of Powder Metallurgy. 1967;3(4): 7-13.
- Hayes GD. Food Engineering Data Handbook. New York: Longman Scientific and Technical. 1987.
- Official Methods of Analysis (14th ed). Association of Official Analytical Chemists, Washington D. C (14th ed.) 1984
- Sani D, Sanni S, Ngulde SI.Effect of intake of aqueous stem extract of Anisopusmannii on haematological parameters in rats. International Journal of Applied Research in Natural Products. 2009;2(3): 22-28.
- Organization for Economic Cooperation and Development (OECD). 1998.
- Sani D, Sanni S, Sandabe UK, Ngulde SI. Toxicological studies on Anisopusmannii crude aqueous stem extract: biochemical and histopathological effects in albino rats. International Journal of Pharmaceutical Sciences. 2010;2(1): 51-59.
- Drury RAB, Wallingthon EA, Camerron SR. (1976). Carlton Histological Techniques.4th ed. Oxford University press, London. 1976; 21-70
- Carlton HM. Carlton’s histological technique, 5th edition Churchill Livingstonr Edinburgh. 1980.
- Fitzpatrick J, Hodnett M, Twomey M, Cerqueira PS, O’flynn J. Glass transition and the flowability and caking of powders containing amorphous lactose. Powder Technology. 2007; 178: 119-128.
- Borah P. 8 Alkaline Foods You Should Include in Your Daily Diet. NDTV food.
- Pandey M, Abidi AB, Singh S, Singh RP. Nutritional evaluation of leafy vegetable paratha.Journal of Human Ecology. 2006; 19(2): 155-156.
- Niranjan RM, Kanaki S. Phytochemical standardization of herbal drugs and poly herbal formulations. Bioactive Molecules and Medicinal Plants. 2008;349-369.
- Perez MF, Flores RA. Particle size of spray dried soymilk. Applied Engineering in Agriculture. 1997;13(5): 647-652.
- Johason JR. Particle segregation and what to do about it.ChemEng.1978;85(2): 183-188.
- Holson JF, Stump DG, Clevidence KJ, Knapp JF, Farr CH. Evaluation of the prenatal developmental toxicity of orally administered arsenic trioxide in rats. Food and Chemical Toxicology. 2000;38(5): 459-466.
- Cavanaugh BM. Nurse’s Manual of Laboratory and Diagnostics Tests.4thedn. FA. Davis Company, Philadelphia, Pa, USA. 2004.
- Johnson-Delaney C. Exotic animal companion medicine handbook for veterinarians. Zoological Education Network. 1996;1(6):15-22.
- Mukinda J, Syce J. Acute and chronic toxicity of the aqueous extract of Artemisia afra in rodents. Journal of Ethnopharmacology. 2007;112(1): 138-144.
- Ramaiah SK. Preclinical Safety Assessment: Current Gaps, Challenges, and Approaches in Identifying Translatable Biomarkers of Drug-Induced Liver Injury. Clinics in Laboratory Medicine. 2011;31(1): 161-172. PMID: 21295728.
- Burtis CA, Ashwood ER, Bruns D. Tietz Fundamentals of Clinical Chemistry, 2001. Saunders Company. Philadelphia, USA.
- Mukinda JT, Eagles PFK. Acute and sub-chronic oral toxicity profiles of the aqueous extract of Polygala fruticosa in female mice and rats. Journal of Ethnopharmacology. 2010;128(1): 236-240.
- Heywood R. Target organ toxicity.Toxicology Letters. 1981, 8(6): 349–358. PMID: 7302965.