Effect of Processing Methods on Nutrient Contents of Sweet Potato (Ipomoea Batatas L. Lam.) Varieties Grown in Ethiopia
Nibret Mekonen*, Henok Nahusenay, Kidist Hailu
Food Sscience and Nutrition Research Directorate, Ethiopian Institute of Agricultural Research (EIAR), Addis Ababa, Ethiopia.
*Corresponding Author
Nibret Mekonen,
Food Sscience and Nutrition Research Directorate, Ethiopian Institute of Agricultural Research (EIAR), Addis Ababa, Ethiopia.
Tel: 0918457605
E-mail: anibretmekonen@gmail.com
Received: March 18, 2022; Accepted: May 14, 2022; Published: May 31, 2022
Citation: Nibret Mekonen, Henok Nahusenay, Kidist Hailu. Effect of Processing Methods on Nutrient Contents of Sweet Potato (Ipomoea Batatas L. Lam.) Varieties Grown in Ethiopia. Int J Food Sci Nutr Diet. 2022;11(3):593-597.
Copyright: Nibret Mekonen© 2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Sweet potato [Ipomoea batatas (L.) Lam.] is an important crop farmed in most of southern and eastern Africa, including Ethiopia,
and is utilized in agriculture, food, and other sectors. The objective of this study was to see how different processing methods
(boiling, frying, roasting, and steaming) altered the proximate composition, vitamin C, and mineral content of four popular
Ethiopian sweet potato cultivars: Tulla, kulfo, Hawassa 83, and Hawassa 09. UV-Vis and AAS methods were used to determine
vitamin C and menial contents, respectively. AOAC methods were used to analyze the proximate composition. The results
revealed that there were significant (p<0.05) differences in crude protein and CHO between cultivars. Total carbohydrate between
varieties ranged from 45.49 to 89.28%, crude fiber (2.08 to 2.51%), crude protein (1.95 to 8.31%), fat (0.45 to 0.85%),
ash (3.88 to 4.23%), and moisture (5.50 to 10.4%). Boiling, roasting, steaming, and frying sweet potato cultivars had no discernible
effect on the crude protein and ash content. However, there was a statistically significant (p<0.05) difference in vitamin C
levels between roasting and other processing methods. Furthermore, there is a significant variation in calcium and potassium
levels (p<0.05) between the kinds. The findings revealed that there is no requirement to select processing methods that result
in the least amount of nutritional loss. This means that the nutritional content of sweet potato types is better preserved after
processing.
2.Introduction
3.Materials and Methods
4.Results and Discussions
5.Conclusions
6.References
Keywords
Minerals; Proximate Composition; Sweet Potato; Vitamin C.
Introduction
Sweet potato [Ipomoea batatas (L.) Lam.] is a key crop in most eastern
and southern African countries, including Uganda, Rwanda,
Kenya, Tanzania, Ethiopia, Zambia, Mozambique, and South Africa
[7]. Sweet potatoes are the world's seventh most important
food crop and the world's second-largest tuber crop, behind Irish
potatoes. It produces 124 million tons per year. It trails only Irish
potato and cassava in terms of acreage (9.1 million ha) among
root and root crops. Sweet potato is Africa's second-largest root
crop after cassava, with production concentrated in East Africa
[10].
For at least 20 million Ethiopians, sweet potatoes are one of the
most essential crops. In terms of sweet potato production, Ethiopia
is ranked fifteenth [14]. In 2010, Ethiopia produced 736,000
MT of sweet potatoes, the highest year in FAOSTAT records and
the ninth most among African countries. The majority of sweet
potatoes are grown in Ethiopia's southern and eastern areas.
White-fleshed sweet potatoes are a staple diet for the Southern
Regional State's 13 million residents. All of Ethiopia's sweet potato
roots are consumed in the domestic food supply, according
to the FAOSTAT study [5].
In the context of African cropping systems, sweet potato has several
advantages: I it produces food in a relatively short period of
time, ii) it yields reliably in sub-optimal growth conditions, iii) it
requires lower labor inputs (suitable for vulnerable households),
v) it serves as an alternative food source for urban populations
facing rising cereal prices, and v) it provides a potential option to
reduce vitamin A deficiency [17].
Antioxidants, fiber, zinc, potassium, sodium, manganese, calcium,
magnesium, iron, vitamin C, and -carotene are all found in sweet
potatoes [8, 11].
Vitamin A insufficiency is a public health issue in Ethiopia, as it is
in other countries of Sub-Saharan Africa. Vitamin A insufficiency
can cause child and mother deaths, as well as a compromised immune
system and blindness. Depending on the variety, 100g of
sweet potato can give anywhere from 0 to 100% of the daily vitamin
A requirement, which is at least 350 g for newborns and 400
g for early children (1-6 years) [6]. Sweet potato, despite its high
carbohydrate content, has a low glycemic index due to the starch's
limited digestion, making it good for diabetics and persons who
are overweight [3, 4].
Sweet potatoes are commonly consumed in Ethiopia by boiling,
steaming, roasting, or frying them [5]. It is vital to obtain knowledge
about the loss of nutrients in various processing processes
in order to make effective use of nutrients from sweet potatoes.
As a result, the goal of this research is to find out how nutrients
vary between cultivars and how different processing methods affect
nutrient loss.
Materials And Methods
Collection and preparation of samples
A total of four varieties of fresh sweet potato, namely Kulfo (yellow),
Tulla (yellow), H-83 (white) and H-09 (white) were collected
from the Hawassa Agricultural Research Center. The collected
samples of fresh sweet potato varieties were washed with clean
tap water and rinsed with distilled water. The peeled and unpeeled
sweet potatoes were cut into pieces and cooked using the following
methods:
Raw (control): Samples were peeled using a kitchen knife, cut
into cubes of about 2.5 cm, washed using distilled water, and then
ground using a mortar and pestle, ready for crude protein, crude
fat, crude fiber, moisture, ash, and mineral content analyses.
Boiling (moist heat): 600 g of unpeeled fresh sweet potato was
rinsed in distilled water, immersed in 750 mL water, and cooked
for 45-55 minutes in a covered saucepan.
Roasting: Unpeeled sweet potatoes were roasted for 20-22 minutes
on hot charcoal, with the sample being moved frequently to
ensure equal roasting.
Steaming: Wrapped in banana leaves, unpeeled sweet potatoes
were cooked for 55-60 minutes.
Frying: Manual peeling with a kitchen knife was used, as was
mechanical chipping with a chipping machine and deep oil frying
with vegetable oil at 140 to 150°C for 10 to 12 minutes.
Proximate analysis
The proximate analysis of both fresh (raw) and processed sweet
potato variety samples was performed in triplicate using the
AOAC 2005 protocol.
Determination of moisture content: Using a 202-1B drying
oven at 105°C for 1 hour, the moisture content of maize cultivars
was evaluated using the AOAC (2005) 925.10 technique. 2 g of
pulverized maize sample was placed in a crucible and dried for
one hour at 130°C, then chilled in a desiccator at room temperature
before being weighed.
% Moisture content = (Weight loss of maize/ Weight of the
orginal maize) × 100
Determination of ash content: Ash content was determined
by the method of AOAC (2005) 923.03 using box-type resistance
(SX2-4-1 OGJ) muffle furnace at 550oC for overnight.
%Ash content = (Weight of ash/ Weight of the orginal maize)
× 100
Determination of fat content: Using a soxtecTM 8000 extraction
device, the AOAC 920.39 technique was used to determine
the crude fat content. To prevent sample loss, three grams of
ground sample were weighed into the soxtec extraction thimble,
and cotton was utilized as a stopper. The aluminum cups with
thimbles were placed in the Soxtec extraction machine, which was
then filled with 50 mL of petroleum ether. The fume hood's water
temperature, water flow rate, and flow rate were all set correctly.
For boiling, rising, and recovery time, the soxtec extraction time
was modified to 15 minutes, 30 minutes, and 10 minutes, respectively.
The extracted and residual solvents were then weighed after
being dried in an oven and chilled in desiccators.
% Crude fat content = (Extracted fat of maize/ weight of maize
sample) × 100
Determination of crude protein: The Kjeldahl technique was
used to evaluate the crude protein content of maize variety samples
(FOSS Analytical AB 2003). 0.5 g of ground sample was
weighed in a Kjeldahl digestion tube, and 2 Kjeltabs CT 3.5 (or 7
g K2SO4 + 0.210 g CuSO4 x 5H2O + 0.210 g TiO2) were added,
followed by 15 mL of concentrated H2SO4. The combination was
carefully heated for 60 minutes inside the fume hood, then cooled
for 15 minutes. After distillation, the crude protein value was calculated
automatically using the Kjeldahl technique.
Determination of crude fiber: The crude fiber of maize varieties
was determined using the FibertecTM 8000 auto-fibre analysis
system, and the percentage of crude fiber was calculated as follows.
% Crude fiber = (W2 - (W3+C)/W1) × 100
Where, W1 is weight of sample, W2 is weight of (crucible + residue),
W3 is weight of (crucible + ash residue) and C is blank.
Determination of carbohydrate content: Carbohydrate content
was determined by difference, that means 100% other proximate
chemical compositions, using the following formula: Carbohydrate
content (%CHO) = 100 (% crude protein +% fat +% ash
+% moisture content +% fiber).
Vitamin C analysis
The analysis was carried out using [2]. The four sweet potato
kinds were sliced and frozen, as were processed sweet potatoes
(boiled, roasted, fried, and steamed). For further investigation,
the frozen samples were freeze-dried and crushed into fine powder
before being stored in a freezer at -20°C. Each of the 0.25 g freeze-dried samples was extracted with 10 mL of 3% (w/v) metaphosphoric
acid and 30 minutes of shaking at 300 rpm and the
extract was centrifuged for 10 minutes at 4000 rpm. The supernatant
was taken and used for further investigation. In 3 percent
(w/v) metaphosphoric acid, a standard curve comprising a series
of known ascorbic acid solutions was produced. 1 mL of either
sample extract or standard substance was added to 3 mL of 0.2
mM DCPIP and measured using UV-Vis at a 515 nm wavelength
after 15 seconds of mixing. The data are given in milligrams of
ascorbic acid per 100 grams of dry weight (mg/100 g DW).
Retention
The apparent retention rate was used to calculate retention. The
ratio of the nutrient content in the cooked food to the nutritional
content in the raw food, given on a dry weight basis, is known as
apparent retention [12].
% Apparent retention = (Nutrient content per g of cooked
food(dry basis)/Nutrient content per g of raw food (dry basis))
× 100
Analysis of mineral content
After dry ashing, the mineral contents (Fe, Zn, Ca, and K) of
each sample were measured by Atomic Absorption Spectrometry
(AAS). 5 mL concentrated HNO3 and 5 mL concentrated HCl
acid solutions were used to digest 0.5 g of each ash sample. The
solution was gently stirred and cooked on a hot plate until yellow
fumes were expelled and it turned clear. After that, a Millipore filter
(0.4) was used to filter the solution and the volume was leveled
to 50 mL with deionized water [1, 15].
Results And Discussion
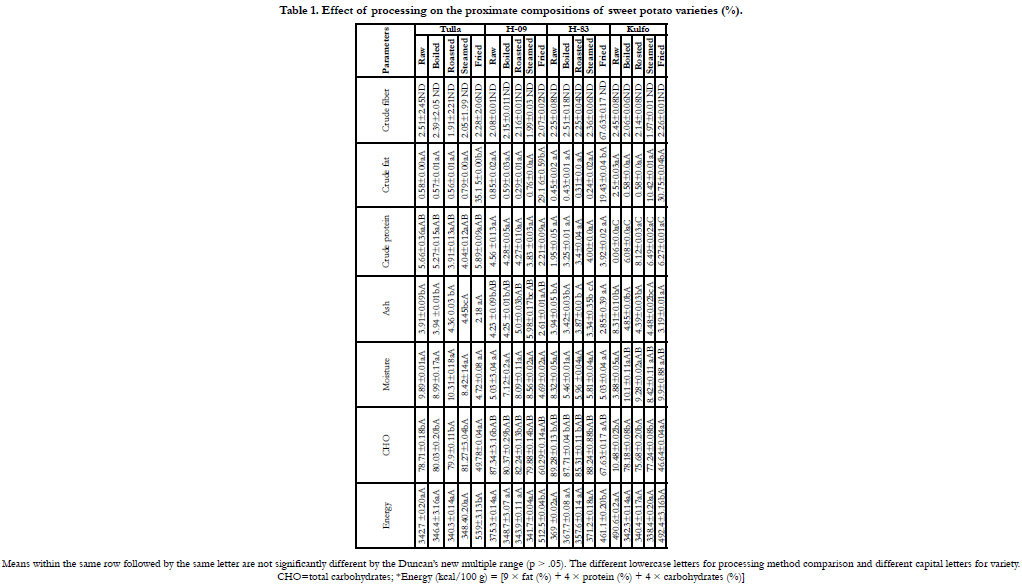
The effects of processing on the proximate compositions of
sweet potato varieties are shown in Table 1. The moisture content
(how much water in the product) was measured in each of the
processed and raw samples of sweet potato varieties. Kulfo (raw)
and kulfo (steamed) had the highest moisture content (10.48%)
and 10.42%, respectively. The minimum amount of moisture
content is 5.5% H-09 (raw) and 4.69% H-09 (fried), respectively.
Ash refers to the remaining or residual parts, mainly inorganic
substances, after the total incineration of organic matter. The ash
content is determined from the loss of weight, which occurs from
the complete oxidation of the sample at a high temperature of
550°C ± 3°C. The ash content for raw and processed sweet potato
varieties ranged from 2.18 to 5.98%.
Fat is an extractable matter from extraction with a specific solvent
like n-Hexane. Crude fat is a mixture of crude fat and soluble material
in the sample that provides energy in the body. The value of
crude fat in sweet potato varieties in raw and processing found to
be from 0.24 to 35.15%. Proteins are made up of many building
blocks, known as amino acids and second ranked proximate composition
next to carbohydrate [16]. The amount of crude protein
in sweet potato varieties ranged from 1.95 to 8.31%. Fiber
(roughage) is the part of plant-based food such as grains, fruits,
vegetables, nuts and beans that the body cannot break down. The
amount of crude fiber found in sweet potato varieties ranged
from 1.91 to 2.51%. In general, the proximate composition of
processing sweet potato varieties are in good agreement with [9].
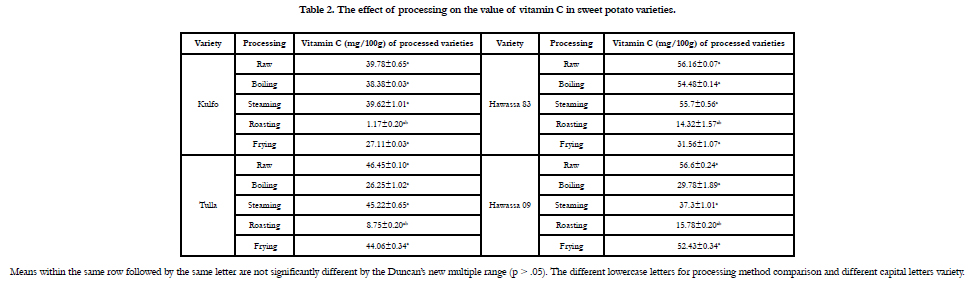
The effect of processing on the value of vitamin C in sweet potato
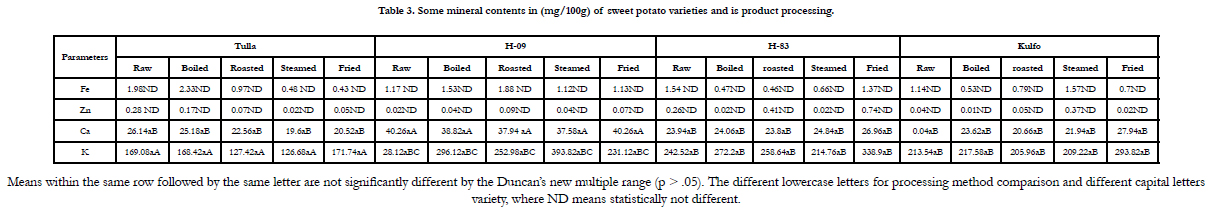
varieties is also represented inTable 2. The mineral contents
such as Fe, Zn, Ca and K in mg/100g are resented in Table 3.
Potassium (K (mg/100g)) was found to be highest than others in
sweet potato varieties and zinc (Zn (mg/100)) was found to be
lowest the others. [13]Have reported the values of iron, zinc, calcium
and potassium in sweet potato varieties were found to be in
the range of 0.25 to 0.73, 0.11 to 0.27, 24.00 to 29.97 and 300 to
326.67 mg/100g, respectively, which is in good agreements in the
present study. Generally, these sweet potato varieties have good
nutritional compositions.
The result of vitamin C in sweet potato varieties and processed
are presented in Table 2. The vitamin C in mg/100g of Hawassa
83 and Hawassa 09were found to be 56.16 and 56.60, respectively.
There is no significant difference in the value of vitamin C on
different processing methods, but there is a significant difference
between roasting and other methods.
Dry matter
Tulla, kulfo, Hawassa 83, and Hawassa 09 sweet potatoes were
studied for dry matter content. Tulla, kulfo, Hawassa 83, and Hawassa
09 sweet potato types have dry matter content of 20.00,
19.45, 31.67, and 24.52, respectively. Hawassa 83 had the highest
dry matter value, whereas Kulfo had the lowest.
Statistical Analysis
The data was analyzed using the Statistical Package for Social
Sciences (SPSS) version 20.0. The descriptive statistics mean
and standard deviation (SD) were calculated, and the data was
reported as mean ± SD. Duncan's new multiple range and twoway
ANOVA were used to compare the means statistically. At a
p<0.05 level, differences in means will be considered significant.
Conclusion
This study compares the nutritional composition of sweet potato
cultivars before and after various processing methods (boiling
(immersed in water and boiled), roasting (roasted over hot charcoal),
steaming (wrapped in banana leaves and boiled), and frying
(wrapped in banana leaves and fried) (deep oil frying with vegetable
oil). The calcium and potassium content of the types differed
significantly (p<0.05) between them, according to the findings
of this study. Boiling, steaming, roasting, and frying sweet potato
cultivars had no significant (p>0.05) effect on ash and crude fiber
content. Within the variations, there is a large variability in calcium
and potassium levels. On the other hand, there was a significant
difference in vitamin C value (p<0.05) between roasting and
other processing methods, and the frying procedure also altered
crude fat content. In addition, it was shown that the nutritional
makeup of sweet potato cultivars retains better after processing.
Acknowledgments
The Ethiopian Institute of Agricultural Research's directorate of
food science and nutrition research has provided financial assistance
for this project. The author wishes to convey their heartfelt
gratitude for their assistance and contribution to the effective completion of this article.
References
- Akinyele IO, Shokunbi OS. Comparative analysis of dry ashing and wet digestion methods for the determination of trace and heavy metals in food samples. Food Chem. 2015 Apr 15;173:682-4. PubMed PMID: 25466076.
- Boonkasem P, Sricharoen P, Techawongstein S, Chanthai S. Determination of ascorbic acid and total phenolics related to the antioxidant activity of some local tomato (Solanum lycopersicum) varieties. Der Pharma Chemica. 2015;7(4):66-70.
- Ellong EN, Billard C, Adenet S. Comparison of physicochemical, organoleptic and nutritional abilities of eight sweet potato (Ipomoea batatas) varieties. Food Nutr Sci. 2014 Jan 14;2014.
- Fetuga G, Tomlins K, Henshaw F, Idowu M. Effect of variety and processing method on functional properties of traditional sweet potato flour ("elubo") and sensory acceptability of cooked paste ("amala"). Food SciNutr. 2014 Nov;2(6):682-91. PubMed PMID: 25493186.
- Jones D, Gugerty MK, Anderson CL. Sweet Potato Value Chain: Ethiopia. Gates Open Res. 2019 Mar 7;3(729):729.
- Kapinga R, Lemaga B, Ewell P, Zhang D, Tumwegamiire S, Agili S, et al. Increased promotion and evaluation of high β carotene sweetpotato as part of the food based approaches to combat Vitamin A deficiency in sub-Saharan Africa (SSA). International Potato Center (CIP) and PRAPACE. 2010.
- Kivuva BM, Musembi FJ, Githiri SM, Yencho CG, Sibiya J. Assessment of production constraints and farmers preferences for sweet potato genotypes. J Plant Breed Genet. 2014 Apr 29;2(1):15-29.
- Laurie SM, Van Jaarsveld PJ, Faber M, Philpott MF, Labuschagne MT. Trans- β-carotene, selected mineral content and potential nutritional contribution of 12 sweetpotato varieties. J Food Compos Anal. 2012 Sep 1;27(2):151-9.
- Lyimo ME, Gimbi DM, Kihinga T. Effect of processing methods on nutrient contents of six sweet potato varieties grown in lake zone of Tanzania. Tanzan J Agric Sci. 2010;10(1).
- Markos D, Loha G. Sweet potato agronomy research in Ethiopia: Summary of past findings and future research directions. Agric Food Sci Res. 2016;3(1):1-1.
- Oloo BO, Shitandi AA, Mahungu S, Malinga JB, Ogata RB. Effects of lactic acid fermentation on the retention of β-carotene content in orange fleshed sweet potatoes. Int J Food Stud. 2014 Apr 18;3(1).
- Rodriguez-Amaya DB, Kimura M. HarvestPlus handbook for carotenoid analysis. Washington: International Food Policy Research Institute (IFPRI); 2004.
- Sanoussi AF, Adjatin A, Dansi A, Adebowale A, Sanni LO, Sanni A. Mineral Composition of Ten Elites Sweet Potato (Ipomoea Batatas [L] Lam) Landraces of Benin. IntJ CurrMicrobiolAppl Sci. 2016;5(1):103-15.
- Tofu A, Anshebo T, Tsegaye E, Tadesse T. Summary of progress on orangefleshed sweet potato research and development in Ethiopia. InProceedings of the 13th ISTRC Symposium 2007 Nov (pp. 728-731).
- Uddin AH, Khalid RS, Alaama M, Abdualkader AM, Kasmuri A, Abbas SA. Comparative study of three digestion methods for elemental analysis in traditional medicine products using atomic absorption spectrometry. JAnalySciTechnol. 2016 Dec;7(1):1-7.
- Ullah I, Ali M, Farooqi A. Chemical and nutritional properties of some maize (Zea mays L.) varieties grown in NWFP, Pakistan. Pak JNutr. 2010;9(11):1113-7.
- Watkins JL, Pogson BJ. Prospects for carotenoid biofortification targeting retention and catabolism. Trends Plant Sci. 2020 May 1;25(5):501-12.