A Novel Approach to Pain Management for the Nuss Procedure using Erector Spinae Plane Blockade and Cryoanalgesia
Casey R Stondell1*, Sundeep S. Tumber DO1, Gary W. Raff2,3, Amy L. Rahm2,3
1 Department of Anesthesiology, Shriners Hospitals for Children Northern California, Stockton Boulevard, Sacramento, CA, USA.
2 Department of Surgery, Shriners Hospitals for Children Northern California, Stockton Boulevard, Sacramento, CA, USA.
3 Department of Cardiothoracic Surgery, University of California Davis Medical Center, Stockton Boulevard, Sacramento, CA, USA.
*Corresponding Author
Dr. Casey R. Stondell M.D.,
Department of Anesthesiology, Shriners Hospitals for Children Northern California,
2425 Stockton Boulevard, Sacramento, 95817, USA.
Tel: 530-304-6034
Fax: 916-453-2047
E-mail: cstondell@shrinenet.org
Received: February 05, 2020; Accepted: February 25, 2020; Published: February 27, 2020
Citation: Casey R Stondell, Sundeep S. Tumber DO, Gary W. Raff, Amy L. Rahm. A Novel Approach to Pain Management for the Nuss Procedure using Erector Spinae Plane Blockade and Cryoanalgesia. Int J Anesth Res. 2020;7(4):584-588. doi: dx.doi.org/10.19070/2332-2780-20000116
Copyright: Casey R Stondell© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Nuss bar placement results in severe and prolonged postoperative pain [1, 2]. Cryoanalgesia (cryo) is a promising new approach for managing thoracic pain which involves freezing intercostal nerves, resulting in analgesia of the chest wall for months [3, 4]. However, the analgesic effect of cryo is delayed for 24 hours or more [5, 6]. Erector Spinae Plane Blockade (ESPB) provides immediate analgesia to the chest wall and can act as an analgesic bridge until cryo reaches peak effect [7, 8].
Methods: We reviewed the charts of all patients who underwent Nuss bar placement between June and July of 2018. Patients received multimodal and preemptive analgesia as well as bilateral thoracic ESPB and cryo. Patient demographics, analgesic techniques, opioid consumption, pain scores, length of stay (LOS), and antiemetic administration were recorded.
Results: Analysis included 1 female and 6 male patients. Mean age was 14.9 +/- 1.9 years and mean Haller index was 3.60 +/- 0.73. Mean LOS was 1.34 +/- 0.56 days. Mean intraoperative fentanyl administration was 5.3 +/- 1.25 mcg/kg. Mean long-acting opioid consumption was 0.43 +/- 0.38 mg/kg PO morphine equivalents on POD 0 and 0.51 +/- 0.73 mg/kg PO morphine equivalents on POD 1. Mean visual analog scale (VAS) pain scores were 2.5 +/- 2.3 and 3.0 +/- 0.97 for POD 0 and 1, respectively. Two patients (28.6%) required antiemetic treatment.
Conclusions: This small historical cohort study shows that it is possible to have short LOS and good pain control after the Nuss procedure by using a combination of ESPB, cryo and multimodal analgesia.
2.Introduction
3.Methods
4.Statistical Analysis
5.Results
6.Discussion
7.Acknowledgments
8.References
Keywords
Anesthesia; Regional; Cryoablation; Intercostal Nerves; Nonopioid Analgesics; Pain; Postoperative; Neuralgia.
Introduction
Pectus excavatum is the most common chest wall deformity in children [1, 5]. Its repair requires a sub-sternal metal bar to be placed across the thoracic cavity, exerting outward pressure on the chest wall, thereby correcting the concave deformity. The minimally invasive placement of this bar via a thoracoscopic approach, known as the Nuss procedure, has become the techniqueof choice for repairing pectus excavatum. Although this does result in good cosmetic repair, it also causes severe and prolonged postoperative pain. Optimal pain management remains elusive, making early discharge after the procedure challenging [4].
Multimodal analgesia has become a mainstay of pain management and should be a standard part of managing patients undergoing the Nuss procedure [9]. In addition to oral and intravenous (IV) medications, regional anesthesia is often required. Techniques commonly utilized include thoracic epidurals (TE) and paravertebral nerve blocks (PVB). TE has been shown to control postoperative pain well but there are considerable disadvantages including risk of neurologic injury, need for prolonged bladder catheterization, pruritus and nausea, delayed ambulation, and difficulty in transitioning from epidural to IV or oral analgesics [9, 10]. PVBs have been shown to provide good postoperative analgesia after Nuss bar placement and they may have a lower risk of neurologic injury when compared to TEs, but performing a PVB is technically challenging and requires needle placement very close to the pleura, increasing the risk of block failure and pneumothorax [1].
Erector Spinae Plane Blockade (ESPB) was first described by Forero in 2016 as a way of managing intractable thoracic neuropathic pain [11]. Local anesthetic is injected just deep to the erector spinae muscle resulting in analgesia to the chest wall, presumably from action on the ventral and dorsal rami of spinal nerves, and possibly due to some epidural spread, although the exact mechanism of action is unknown [12, 13]. ESPB is simple to perform and may be safer than TE or PVB [7, 8]. Multiple studies have shown the efficacy of ESPB for thoracic analgesia and, when considering that it may have a better safety profile than other regional or neuraxial techniques, it may be the ideal block for Nuss bar placement [14].
All of the techniques listed above have the potential to successfully manage immediate postoperative pain after Nuss bar placement, but the pain from this procedure can last for weeks [2, 4]. A promising approach for providing long-term thoracic analgesia is emerging in the form of cryoanalgesia (cryo), also known as cryoablation, which involves freezing intercostal nerves, resulting in thoracic analgesia that usually lasts months [3]. In 2016 Keller published one of the first papers examining the use of cryo for Nuss bar placement and found that utilizing cryo led to reduced time to hospital discharge and decreased opioid consumption when compared to TE [15]. While cryo does seem to work well for treating thoracic pain, peak analgesic effect does not take effect for 24 hours or more; hence, another intervention is needed for managing immediate postoperative pain [5, 6].
We began performing ESPB in patients undergoing the Nuss procedure in an effort to provide an analgesic bridge from the time of surgery to the time of peak cryo effect. We then retrospectively reviewed these cases, hypothesizing that the combination of ESPB and cryo would result in short LOS, good pain control, and low opioid consumption.
Methods
After IRB approval (IRB NCA1711R), we retrospectively reviewed the charts of all patients who underwent Nuss bar placement for correction of pectus excavatum in June and July of 2018. Data from 7 charts were collected and included patient demographics, Haller indexes, medication administration, operative details, anesthetic management, visual analog scale (VAS) pain scores, presence of postoperative nausea or vomiting (defined as the need for postoperative antiemetic medication) and LOS.
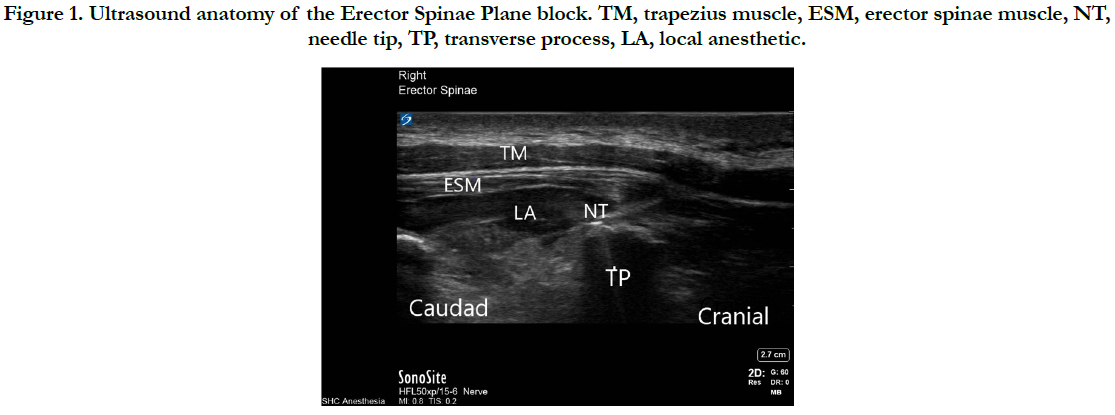
All patients were managed with a standardized perioperative multimodal analgesic regimen including preoperative celecoxib 200mg, gabapentin (12.5-15mg/kg up to 900mg) and either preoperative or intraoperative acetaminophen (12.5-15mg/kg up to 1,000mg). After induction of general anesthesia and endotracheal intubation, bilateral ESPB was performed. With the ultrasound probe oriented cranial to caudad, transverse processes were identified at approximately the T6 level. A needle was then advanced towards the transverse process just deep to the erector spinae muscle. After confirming adequate needle placement with a 2ml bolus of normal saline, 20ml of 0.2% ropivacaine with epinephrine 1:200,000 was injected. Detailed ultrasound anatomy of the ESPB is displayed in Figure 1.
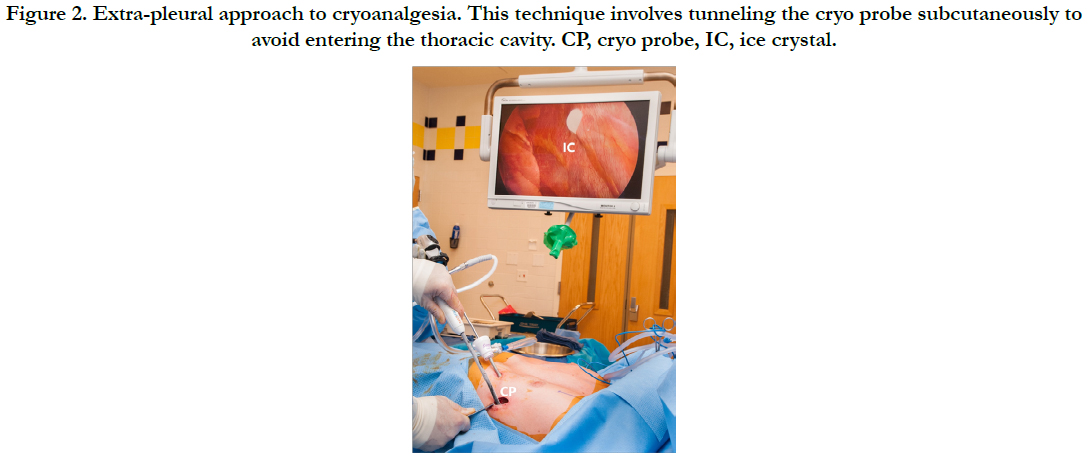
Cryo was then performed by the surgeon under thoracoscopic guidance using an extra pleural technique. A cryo probe (Atricure, Inc.) was inserted into the chest wall, utilizing the same incision later used for Nuss bar insertion. Surgeons then tunneled the probe subcutaneously, avoiding violation of the pleura. The probe was advanced through the subcutaneous tissue and brought into contact with the intercostal nerve of the third, fourth, fifth, sixth and seventh intercostal spaces bilaterally and cooled to -60 degrees Celsius (°C) for two minutes. Thoracoscopic visualization of ice crystal formation, as seen in Figure 2, as well as intercostal muscle fasciculations confirmed appropriate placement. After cryo completion, the Nuss bar was placed under thoracoscopic guidance. No additional local anesthetic was injected by surgeons. Patients were given intraoperative IV hydromorphone and fentanyl per the anesthesiologist’s discretion. All patients were extubated at the end of the procedure without complication.
Postoperatively, all patients received scheduled acetaminophen (15mg/kg up to 1,000mg Q6 hours), NSAIDs (IV ketorolac 0.5mg/kg max 30mg Q6 hours, switched to PO Ibuprofen 10mg/ kg up to 600mg once tolerating orals) and gabapentin 300mg PO QHS. The gabapentin was continued until chest wall sensation returned to normal, usually about 2-3 months after cryo was performed. Opioid pain medications were ordered per the surgeon’s discretion. Four patients were written for PRN pain medications only, while 3 patients received demand-only patient controlled analgesia (PCA). Patients were discharged to home after their pain was controlled on PO analgesics and they were ambulating and tolerating a regular diet.
Statistical Analysis
Means and standard deviations were calculated for all quantitative variables and proportions were calculated for categorical variables. Each patient received IV fentanyl throughout the case per the anesthesiologists’ discretion. Intraoperative fentanyl was not included in total opioid consumption calculations because it is unlikely to have a significant effect on postoperative opioid consumption or length of stay due to its short half-life. IV hydromorphone was the only long-acting opioid used intraoperatively and was included in the opioid consumption data. PO morphine equivalents were calculated based on the formula in Table 1.
Results
Seven patients underwent Nuss bar placement in June and July of 2018 and all were included in the analysis. All but one of the study patients were male. Mean patient age was 14.9 +/- 1.9 years and mean Haller index was 3.60 +/- 0.73. All patients were ASA 1 or 2. Mean intraoperative fentanyl administration was 5.3 +/- 1.25 mcg/kg. Mean long-acting opioid consumption was 0.43 +/- 0.38 mg/kg PO morphine equivalents on post-operative day (POD) 0, including intraoperative hydromorphone, and 0.51 +/- 0.73 mg/ kg PO morphine equivalents on POD 1. Mean LOS was 1.34 +/- 0.56 days. Mean VAS pain scores were 2.5 +/- 2.3 and 3.0 +/- 1 for POD 0 and 1, respectively. 2 patients (28.6%) experienced postoperative nausea requiring pharmacologic treatment.
Discussion
In our small historical cohort study we found that by combining ESPB with cryo for the Nuss procedure, patients had low pain scores, low opioid consumption, and short LOS. Although we do not have a comparison group in our study, a recent observational study by Muhly et al., as part of the Society for Pediatric Anesthesia Improvement Network (SPAIN), analyzed patients undergoing the Nuss procedure at 14 different institutions managed with TEs, PVBs, wound catheters, or no regional [16]. A total of 331 patients were analyzed, none of whom received cryo. Average POD 0 and POD 1 pain scores and opioid consumption were similar to our study. Muhly et al., found that under 10% of patients from any group were discharged by POD 2 and under 50% of patients from any group were discharged by POD 3. In our study the average LOS was 1.34 days and all patients were discharged by POD 2. We provide the above comparison simply to show that, in our study, LOS seems to be shorter than that typically seen with Nuss bar placement while achieving pain scores and opioid consumption similar to that seen with other pain interventions.
Cryo has been used for decades to treat chronic and acute pain but its use in pediatrics, and specifically its use for the Nuss procedure, is quite new [4-6, 17]. The extent and duration of analgesia following cryo, as well as the risk of nerve injury, are dependent on the temperature of the cryoprobe and the duration of cryo application. Zhou et al., examined the clinical and histologic effects of various temperatures of cryo application to the sciatic nerve in rabbits [18, 19]. Nerve morphology, somatosensory evoked potentials (SSEPs), and motor function, as indicated by weakness or foot drop, degraded as colder temperatures were applied, implying a direct link between temperature and degree of nerve injury and recovery. They concluded that temperatures between -60°C and -100°C may be optimal for providing analgesia while minimizing the risk of persistent neurologic issues.
Moorjani et al., investigated how the duration of cryo application affects the structure and function of peripheral nerves. Canine intercostal nerves were cooled to -50°C for various periods of time, with no evident long-term histologic damage after application up to 120 seconds [20]. In a separate arm of the study, 200 human subjects undergoing thoracotomy were randomized to receive either cryo at -50°C for 60 seconds or conventional therapy. Patients in the cryo group had lower pain scores, lower opioid consumption and improved pulmonary function testing compared to the control group and had complete neurologic recovery within 6 months. The study concluded that cryo seems to be safe and effective, yet the theoretical risk of post-cryo neuralgia remains.
The incidence of post cryo neuropathic pain is unclear, but based on current literature it appears to be low [20-23]. To minimize this risk, at our institution we administer a large preoperative loading dose of gabapentin as well as scheduled postoperative gabapentin, which is continued until patients have return of normal chest wall sensation, typically 2 to 3 months. We have performed over 65 cryo treatments and have had no reported cases of neuralgia.
Another potential pitfall of using cryo for analgesia after the Nuss procedure is the time it takes to reach peak effect. Multiple studies have shown that peak analgesia after cryo is delayed for 24 hours or more and that another form of analgesia is needed as a bridge during that time [5, 6]. Some have used TE, PVB, or even extended-release oxycodone to form this bridge, but ESPB may be a better alternative. ESPB has been shown to be a safe and effective means of providing analgesia for up to 24 hours after a single injection, and it may be safer and easier to perform than TE or PVB [7, 8, 11, 14]. Continuous ESPB catheters have been used to provide analgesia after the Nuss procedure in the past but in our experience, with the addition of cryo, catheters become unnecessary [24, 25]. To our knowledge our study if the first to combine single shot ESPB with cryo for the Nuss procedure.
Our study does have several limitations, including being retrospective in nature, not having a comparison group, and having a small sample size. However, we show that it is possible to have good pain control and short length of stay after Nuss bar placement by using the novel technique of combining ESPB with cryo and multimodal analgesia.
We suggest that ESPB may be the optimal way to manage immediate postoperative thoracic pain in this population because it is easy to perform, safe and effective, and provides a non-opioid based foundation for pain management [7, 8]. Likewise, cryo may be the optimal way to minimize long-term postoperative opioid usage by providing long-lasting pain relief from a single treatment. In order to better assess whether the results in our study are more broadly applicable, prospective studies with larger sample sizes are needed.
Acknowledgements
We wish to thank Sandra Taylor, Principal Statistician at the Clinical and Translational Science Center in Sacramento, California for assisting with statistical calculations and data analysis. We also wish to thank Sampaguita Tafoya, MD, for assistance in editing the manuscript.
References
- Hall burton DM, Boretsky KR. A comparison of paravertebral nerve block catheters and thoracic epidural catheters for postoperative analgesia following the Nuss procedure for pectus excavatum repair. Paediatr Anaesth. 2014 May;24(5):516-20.Pubmed PMID: 24612096.
- Kim S, Idowu O, Palmer B, Lee SH. Use of transthoracic cryoanalgesia during the Nuss procedure. J Thorac Cardiovasc Surg. 2016 Mar;151(3):887- 888. Pubmed PMID: 26896363.
- Green CR, De rosayro AM, Tait AR. The role of cryoanalgesia for chronic thoracic pain: results of a long-term follow up. J Natl Med Assoc. 2002 Aug;94(8):716-20. Pubmed PMID: 12152929.
- Graves C, Idowu O, Lee S, Padilla B, Kim S. Intraoperative cryoanalgesia for managing pain after the Nuss procedure. J Pediatr Surg. 2017 Jun;52(6):920-924. Pubmed PMID: 28341230.
- Sujka J, Benedict LA, Fraser JD, Aguayo P, Millspaugh DL, St peter SD. Outcomes Using Cryoablation for Postoperative Pain Control in Children Following Minimally Invasive Pectus Excavatum Repair. J Laparoendosc Adv Surg Tech A. 2018 Nov;28(11):1383-1386. Pubmed PMID: 29927703.
- Harbaugh CM, Johnson KN, Kein CE, Jarboe MD, Hirschl RB, Geiger JD,et al. Comparing outcomes with thoracic epidural and intercostal nerve cryoablation after Nuss procedure. J Surg Res. 2018 Nov;231:217-223. Pubmed PMID: 30278932.
- Tulgar S, Selvi O, Senturk O, Serifsoy TE, Thomas DT. Ultrasound-guided Erector Spinae Plane Block: Indications, Complications, and Effects on Acute and Chronic Pain Based on a Single-center Experience. Cureus. 2019 Jan 2;11(1):e3815. Pubmed PMID: 30868029.
- Kot P, Rodriguez P, Granell M, Cano B, Rovira L, Morales J, et al. The erector spinae plane block: a narrative review. Korean J Anesthesiol. 2019 Jun;72(3):209-220.Pubmed PMID: 30886130.
- Singhal NR, Jerman JD. A review of anesthetic considerations and postoperative pain control after the Nuss procedure. SeminPediatr Surg. 2018 Jun;27(3):156-160. Pubmed PMID: 30078486.
- Stroud AM, Tulanont DD, Coates TE, Goodney PP, Croitoru DP. Epidural analgesia versus intravenous patient-controlled analgesia following minimally invasive pectus excavatum repair: a systematic review and meta-analysis. J Pediatr Surg. 2014 May;49(5):798-806. Pubmed PMID: 24851774.
- Forero M, Adhikary SD, Lopez H, Tsui C, Chin KJ. The Erector Spinae Plane Block: A Novel Analgesic Technique in Thoracic Neuropathic Pain. Reg Anesth Pain Med. 2016 Sep-Oct;41(5):621-7. Pubmed PMID: 27501016.
- Schwartzmann A, Peng P, Maciel MA, Forero M. Mechanism of the erector spinae plane block: insights from a magnetic resonance imaging study. Can J Anaesth. 2018 Oct;65(10):1165-1166. Pubmed PMID: 30076575.
- Vidal E, Giménez H, Forero M, Fajardo M. Erector spinae plane block: A cadaver study to determine its mechanism of action. Rev Esp Anestesiol Reanim. 2018 Nov;65(9):514-519. Pubmed PMID: 30166123.
- Tsui BCH, Fonseca A, Munshey F, Mcfadyen G, Caruso TJ. The erector spinae plane (ESP) block: A pooled review of 242 cases. J Clin Anesth. 2019 Mar;53:29-34. Pubmed PMID: 30292068.
- Keller BA, Kabagambe SK, Becker JC, Chen YJ, Goodman LF, Clark-Wronski JM, et al. Intercostal nerve cryoablation versus thoracic epidural catheters for postoperative analgesia following pectus excavatum repair: Preliminary outcomes in twenty-six cryoablation patients. J Pediatr Surg. 2016 Dec;51(12):2033-2038. Pubmed PMID: 27745867.
- Muhly WT, Beltran RJ, Bielsky A, Bryskin RB, Chinn C, Choudhry DK, et al. Perioperative Management and In-Hospital Outcomes After Minimally Invasive Repair of Pectus Excavatum: A Multicenter Registry Report From the Society for Pediatric Anesthesia Improvement Network. AnesthAnalg. 2019 Feb;128(2):315-327. Pubmed PMID: 30346358.
- Morikawa N, Laferriere N, Koo S, Johnson S, Woo R, Puapong D. Cryoanalgesia in Patients Undergoing Nuss Repair of Pectus Excavatum: Technique Modification and Early Results. J Laparoendosc Adv Surg Tech A. 2018 Sep;28(9):1148-1151. Pubmed PMID: 29672193.
- Zhou L, Shao Z, Ou S. Cryoanalgesia: electrophysiology at different temperatures. Cryobiology. 2003 Feb;46(1):26-32. Pubmed PMID: 12623025.
- Zhou L, Kambin P, Casey KF, Bonner FJ, O'Brien E, Shao Z, et al. Mechanism research of cryoanalgesia. Neurol Res. 1995 Aug;17(4):307-11. Pubmed PMID: 7477749.
- Moorjani N, Zhao F, Tian Y, Liang C, Kaluba J, Maiwand MO. Effects of cryoanalgesia on post-thoracotomy pain and on the structure of intercostal nerves: a human prospective randomized trial and a histological study. Eur J Cardiothorac Surg. 2001 Sep;20(3):502-7. Pubmed PMID: 11509270.
- Sepsas E, Misthos P, Anagnostopulu M, Toparlaki O, Voyagis G, Kakaris S. The role of intercostal cryoanalgesia in post-thoracotomy analgesia. Interact Cardiovasc Thorac Surg. 2013 Jun;16(6):814-8. Pubmed PMID: 23424242.
- Hunt I, Eaton D, Maiwand O, Anikin V. Video-assisted intercostal nerve cryoablation in managing intractable chest wall pain. J Thorac Cardiovasc Surg2010 Mar;139(3):774-5. Pubmed PMID: 20176220.
- Ba YF, Li XD, Zhang X, Ning ZH, Zhang H, Liu YN, et al. Comparison of the analgesic effects of cryoanalgesia vs. parecoxib for lung cancer patients after lobectomy. Surg Today. 2015 Oct;45(10):1250-4. Pubmed PMID: 25300198.
- Yoshizaki M, Murata H, Ogami-takamura K, Hara T. Bilateral erector spinae plane block using a programmed intermittent bolus technique for pain management after Nuss procedure. J Clin Anesth. 2019 Nov;57:51-52. Pubmed PMID: 30852328.
- Lowery DR, Raymond DP, Wyler DJ, Marciniak DA. Continuous Erector Spinae Plane Blocks for Adult Pectus Excavatum Repair. Ann Thorac Surg. 2019 Jul;108(1):e19-e20. Pubmed PMID: 30597141.