Can Partial Oxygen Pressure Of Urine Be An Indicator For Tissue Perfusion? Review’
Melis TOSUN*
Aksaray Eskil Devlet Hastanesi, Department of Anesthesiology and Reanimation, Aksaray- 68100, Turkey.
*Corresponding Author
Melis TOSUN,
Aksaray Eskil Devlet Hastanesi, Department of Anesthesiology and Reanimation, Aksaray- 68100, Turkey.
Tel: +90-536-6694002
E-mail: melistosun@gmail.com
Received: November 20, 2021; Accepted: January 22, 2022; Published: February 07, 2022
Citation: Melis TOSUN. Can Partial Oxygen Pressure Of Urine Be An Indicator For Tissue Perfusion? Review’. Int J Anesth Res. 2022;10(1):673-675.
Copyright: Melis TOSUN© 2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The main purpose of advanced monitorisation is to detect hypoperfusion before any irreversible damage. However, none
of the advanced monitorisation procedures focusing only on the hemodynamics and blood gas parameters are sufficient to
estimate tissue perfusion adequately. As low urine partial oxygen pressure (PuO2) is an indicator for medullary hypoperfusion,
PuO2 measurement is one of the promising markers for early detection of hypoperfusion. Based on this hypothesis, we had
designed a study to evaluate the use of PuO2 together with routine systemic tissue perfusion parameters in patients undergoing
open-heart surgery with extracorporeal circulation. Fifty patients undergoing elective coronary artery bypass graft surgery had
included. In addition to the routine hemodynamic monitorization; cardiac output (CO), Pv-aCO2, blood, and urine gas analysis
were performed at 180th, 360th, and 540th minutes postoperatively, and creatinine was measured repetitively postoperatively.
None of the patients developed any hemodynamic event related to hypoperfusion or acute renal injury. No correlation between
hemodynamic and blood gas parameters with PuO2 could be shown in this study. In conclusion, further studies on patients
with hypoperfusion and transient renal ischemia may correctly resolve the correlation.
2.Introduction
3.Methodology
4.Results
5.Discussions
6.Conclusion
7.Acknowledgments
8.References
Introduction
Undetected hypoperfusion, improper delivery of oxygen, or impaired
oxygen consumption may lead to irreversible organ damage
or even death. The main purpose of advanced monitorisation
is to detect hypoperfusion before any irreversible damage. However,
none of the advanced monitorisation procedures focusing
only on the hemodynamics and blood gas parameters are sufficient
to estimate tissue perfusion adequately [1]. Limited information
obtained by the routine measurements has led us to look for
new parameters to predict hypoperfusion.
As cardiac surgery with extracorporeal circulation (ECC) is a highrisk
surgery and the end-organ damage secondary to impaired microcirculation
is one of the main determinants of postoperative
mortality, ensuring the adequacy of the microcirculation should
be the main focus. However, the impaired cardiac pressure-volume
relationship of these patients makes the routine evaluation
of tissue perfusion more difficult.
The renal medullary oxygenation is determined by four parameters;
medullary blood flow (MBF), medullary oxygen consumption
rate (V̇ O2,M), hemoglobin (Hb) concentration, and renal
perfusion pressure, and renal medulla is highly sensitive and one
of the first damaged tissues by hypoperfusion [2-4]. It has shown
that urine partial oxygen pressure (PuO2) is altered by renal arterial
flow, hence renal medullary oxygen pressure, and PuO2 sampled
from the collecting tubules is a marker for medullary oxygenation
[5].
As low PuO2 is an indicator for medullary hypoperfusion, PuO2
measurement is one of the promising markers for early detection
of hypoperfusion. Based on this hypothesis, we had designed a
study to evaluate the use of PuO2 together with routine systemic
tissue perfusion parameters in patients undergoing open-heart
surgery with ECC [6].
Fifty patients undergoing elective coronary artery bypass graft
surgery had included. In addition to the routine hemodynamic
monitorization; cardiac output (CO), Pv-aCO2, blood, and urine
gas analysis were performed at 180th (T0), 360th (T1), and 540th
(T2) minutes postoperatively. Urine was collected through a silicone
urine catheter inside the bladder. CO measurement was performed
by using a finger cuff method. Through the ECC, hematocrit
was kept at 23%-30%. The pump flow rate was maintained
>2 L m−1 during ECC, mean arterial blood pressure (ABP) was
kept between 50-80 mmHg. Moderate hypothermia (32°C) was
applied to all patients. To the evaluation of renal functions, serum creatinine was measured perioperatively.
The patients were hemodynamically stable throughout the surgery
and post-extubation period in terms of heart rate, mean arterial
pressure, and CO (p>0.05). Additionally, blood gas analyses
and electrolyte concentrations were also found within the normal
limits (p>0.05).
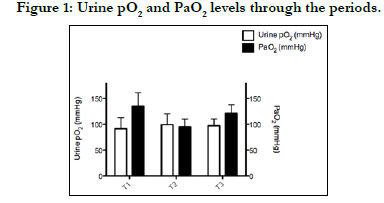
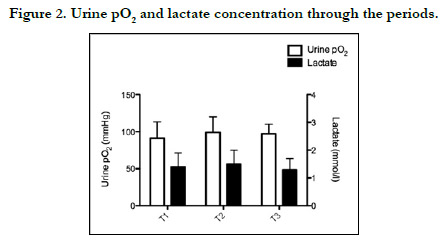
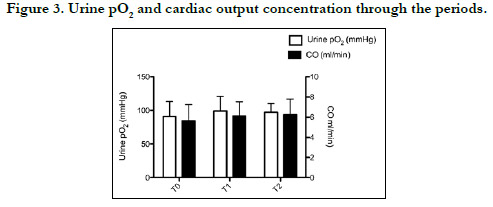
Postoperative PuO2 was measured as 91±22, 99±22, and 97±13
mm Hg, respectively, and was no significant difference at any time
point. Moreover, there were no significant correlations between
PuO2 and other tissue oxygenation parameters (p>0.05) (Figures
1-3).
Serum creatinine was measured preoperatively and postoperative
first and 5th days as 0.92±0.5, 0.93±0.5, and 0.85±0.5 mg dL−1,
respectively, and none of the patients developed acute renal injury
(ARI).
The limited information obtained from routine monitoring methods has encouraged us to look for new monitoring methods. The new technique should be non-invasive, easy to perform, safe, low in price, and capable of detecting the early stages of hypoperfusion. In this concerns, we investigated the correlation of PuO2 with advanced monitoring parameters in a patient group at risk for hypoperfusion.
Although there are animal studies in the literature showing a correlation between cardiac output and mean arterial pressure with PuO2[7], no correlation between hemodynamic and blood gas parameters with PuO2 could be shown in this study.
As we mentioned in the original article, our study had some limitations. First, we compared PuO2 of bladder urine samples instead of renal medullary or pelvic urine. Although bladder urine has been shown to reflect renal medullary oxygenation [8], the PuO2 that might have been pooled in the bladder may vary.
Although Xing et al.[9] studied PuO2 measurements for early diagnosis of AKI in septic patients and found a cut-off value for renal injury to find the normal ranges of renal medullary, renal pelvic, and bladder PuO2 need more studies in larger patient groups. Also, animal studies may help us to find the factors affecting PuO2 while urine passing through the urinary tract.
In our opinion, since the COs of the patients were within the normal limits, and none of the patients developed an ischemic condition or AKI, the present study might have been unable to determine any correlation. Further studies on patients with transient renal ischemia may correctly resolve the correlation. Additionally, up-to-date AKI markers should be measured in the upcoming studies to detect renal damage that cannot be detected by creatinine monitoring.
References
- Clifford PS. Local control of blood flow. Advances in physiology education. 2011 Mar;35(1):5-15.
- Evans RG, Smith DW, Lee CJ, Ngo JP, Gardiner BS. What makes the kidney susceptible to hypoxia?. The Anatomical Record. 2020 Oct;303(10):2544- 52.
- Mullens W, Nijst P. Cardiac output and renal dysfunction: definitely more than impaired flow. Journal of the American College of Cardiology. 2016 May 17;67(19):2209-12.
- Lee CJ, Gardiner BS, Evans RG, Smith DW. Analysis of the critical determinants of renal medullary oxygenation. American Journal of Physiology- Renal Physiology. 2019 Dec 1;317(6):F1483-502.
- Evans RG, Smith JA, Wright C, Gardiner BS, Smith DW, Cochrane AD. Urinary oxygen tension: a clinical window on the health of the renal medulla?. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology. 2014 Jan 1;306(1):R45-50.
- Tosun M, Ulugöl H, Aksu U, Toraman F. Can Partial Oxygen Pressure of Urine be an Indicator for Tissue Perfusion?. Turkish Journal of Anaesthesiology and Reanimation. 2019 Jun;47(3):187.
- Kitashiro SH, Iwasaka TO, Sugiura TE, Takayama YA, Tamura TE, Tamura KO, et al. Monitoring urine oxygen tension during acute change in cardiac output in dogs. Journal of Applied Physiology. 1995 Jul 1;79(1):202-4.
- Valente A, Sorrentino L, La Torre G, Draisci G. Post-transfusional variation in urinary oxygen tension in surgical patients. Clinical and experimental pharmacology & physiology. 2008 Apr 21;35(9):1109-12.
- Xing ZQ, Liu DW, Wang XT, Long Y, Zhang HM, Wang C, Huang W. The value of renal resistance index and urine oxygen pressure for prediction of acute kidney injury in patients with septic shock. Zhonghuaneikezazhi. 2019 May 1;58(5):349-54.