Oral Health Issues in Psoriasis: An Overview of the Literature
Monson CA1*, Silva V2, Porfírio G1, Riera R1, Tweed JA2, Petri V1, Atallah ÁN1
1 Department of Medicine, Escola Paulista de Medicina (EPM), Federal University of São Paulo (UNIFESP), Brazil.
2 Department of Medicine, Centro Universitário Tiradentes (UNIT-AL), Maceió, Brasil.
3 The Cochrane Skin Group, University of Nottingham, UK.
*Corresponding Author
Carlos Alberto Monson,
Department of Medicine, Escola Paulista de Medicina (EPM),
Federal University of São Paulo (UNIFESP), Brazil.
Tel: +55 11 5062-2796/+55 11 9 9569-9824
E-mail: acmonson@uol.com.br
Received: February 29, 2016; Accepted : November 16, 2016; Published: November 19, 2016
Citation: Monson CA, Silva V, Porfírio G, Riera R, Tweed JA, et al., (2016) Oral Health Issues in Psoriasis: An Overview of the Literature. Int J Clin Dermatol Res. 4(4), 94-103. doi: http://dx.doi.org/10.19070/2332-2977-1600025
Copyright: Monson CA© 2016. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Psoriasis and oral health are overlap in different ways, sometimes with complex and uncertain outcomes.
Objective: It is to map the knowledge about the oral health status issues for psoriasis.
Methods: We conducted qualitative systematic review of clinical trials literature.
Results: There are different issues about oral health status related to the observed outcomes. It was recovered 830 studies, 11 were able to fill partially the inclusion criteria for this review with 293.231 participants. The highest level of evidence obtained was 2 A (Randomized Clinical Trial), for the oral psoriasis arthritis forms at TMJ issue with one study. About the periodontal diseases issue was obtained the second highest level of evidence 2B (Clinical Trial Case-Control), with 10 studies.
Limitations: The main limitation was the lack of inclusion of unpublished studies.
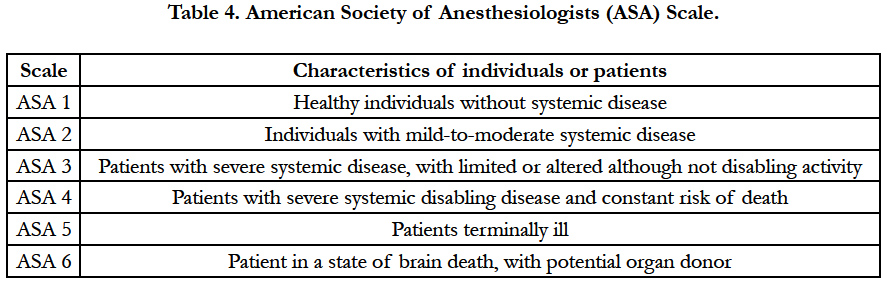
Conclusions: For clinicians It is important consider oral implications for all psoriasis patients. Among possible issues, it is relevant to state that the conventional treatments for psoriasis are able to produce oral adverse events75. Periodontal diseases, seems to be related to the severity of psoriasis, because the level of evidence was only 2B, although its intrinsic mechanisms are not known. Further studies are required to state a minimum dental care protocol. In general, for better outcomes in Psoriasis, professional must adopt an assessment risk scales, such as the “physical status classification system” from the American Society of Anesthesiologists (ASA) and observe statements, such those the American Dental Association, prior to any interventions, concerning to the degree of coverage and invasiveness of each dental procedure and the risk of adverse events before, during and after the consultation for these patients.
2.Introduction
3.Methods
1. Issues related to the pathogenesis of Psoriasis.
2. Issues related to the clinical implications due Psoriasis.
4.Results
5.Discussion
5.1. Issues Related to the Pathogenesis of Psoriasis: It Means That Present Issues are the Oral Manifestations of Disease
5.2. Issues related to the clinical implications due the psoriasis:
6.Conclusion
7.Acknowledgements
8.References
Keywords
Dental Care for Chronically Disease; Drug Utilization; Psoriasis; Morbidity; Risk Factors.
Introduction
Psoriasis is defined as an immune-mediated multiple-cause disease of genetic basis [1] and different phenotypes [2] that affects up to 8.5% of the population in the Western countries and requires careful management [3]. Among the skin chronic forms of psoriasis, those with circumscribed thick white to silver plaques, surrounded by areas redness are more common [4]. Psoriasis can spread to the elbows, knees, shins, scalp, lower back, nails, genitals, mouth and joint areas with many grades of damage, leading to the emotional consequences with negative impact in the patient’s life [3, 6]. Oral cavity can reveals details about systemic health status. Oral diseases also have immune-involvement with multiple causes and can lead to local and systemic complications. Oral care are the health treatments that people more often have performed [74]. Relying on surgical resources and increasingly advanced pharmacological therapy, these treatments can lead to the damages, even in healthy patients [4]. In some conditions, dental treatments can be invasive and for Psoriasis it can cause problems in complex ways. The oral aspects for psoriasis were not assessed with a comprehensive literature review and accurate inferences are possible only if a previous systematic analysis of risk factors was done [5].
Methods
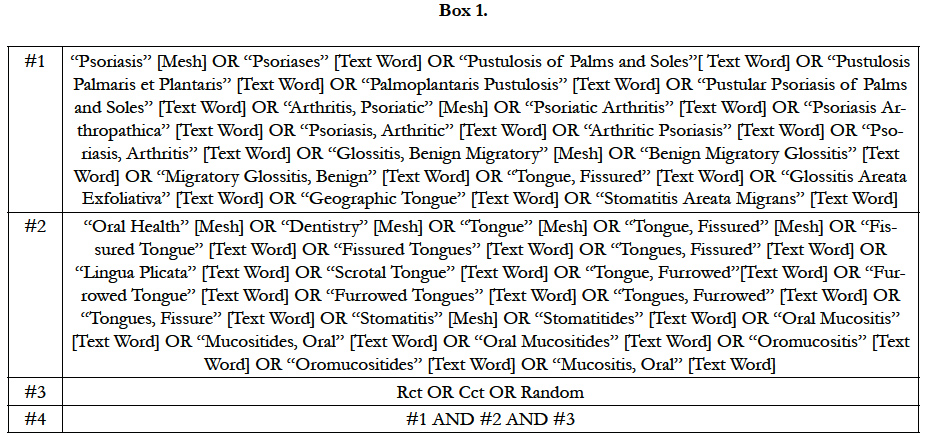
In order to map the knowledge, according the Health Based Evi-dence criteria, a P.I.C.O.T. question was made, “Which are the oral issues for psoriasis? It was observed different issues concerning the theme:
1.1. Oral findings in psoriasis.
1.2. Oral psoriasis arthritis at temporomandibular joint (TMJ).
2.1. Periodontal diseases related to the psoriasis.
2.2. Conventional treatments and oral outcomes.
2.2.1. Inducing oral adverse outcomes.
2.2.2. Worsening oral adverse outcomes.
2.2.3. Inducing oral favorable outcomes.
2.3. Conventional treatments for diseases related to psoriasis inducing oral adverse events.
2.3.1. For Prior Morbidities related to psoriasis.
2.3.2. For Co Morbidities related to psoriasis.
2.3.3. For Post Morbidities related to psoriasis.
2.4. Dental treatments and psoriasis:
2.4.1.Inducing systemic favorable outcomes to psoriasis.
2.4.2.Triggering systemic psoriasis.
2.4.3.Worsening psoriasis.
2.5. Oral risk factors able to induce exacerbation to psoriasis.
It was conducted a systematic review in accordance to the Cochrane Collaboration for Qualitative Research Group (CCQR; Bangor University, Wales, UK) [8] within the Evidence-Based Health Program model at the service (GEPSOS) Grupo de Estudos e Pesquisas Saúde Oral e Sistêmica Baseada em Evidências, Ambulatório Multidisciplinar Psoríase I Departamento Dermatologia, Escola Paulista de Medicina Unifesp in São Paulo, Brazil, and received ethics committee approval from the Ministry of Health of Republic of Brazil. Its prior protocol project was structured based on PRISMA (Preferred reporting items for systematic reviews and meta-analyses). For the assessment of the risk of bias of each included study was used using the criteria described in the Cochrane Handbook for Systematic Reviews of Interventions[8, 9] according to the following domains:
1. Random sequence generation (selection bias);
2. Allocation concealment (selection bias);
3. Blinding of participants and personnel (performance bias);
4. Blinding of outcome assessment (detection bias);
5. Incomplete outcome data (attrition bias);
6. Selective reporting (reporting bias);
7. Other bias (other sources of bias related to a particular trial design, e.g. cross-over or cluster-randomized, or specific circumstances, e.g. interventions mixed).
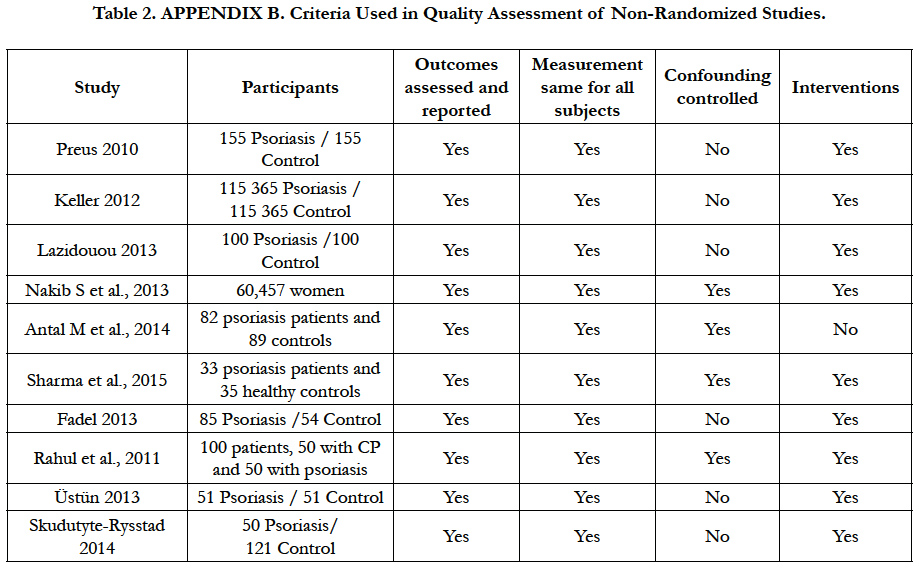
Each of the items is judged as low risk of bias, high risk of bias or unclear risk of bias, for included studies [9] (Table 2).
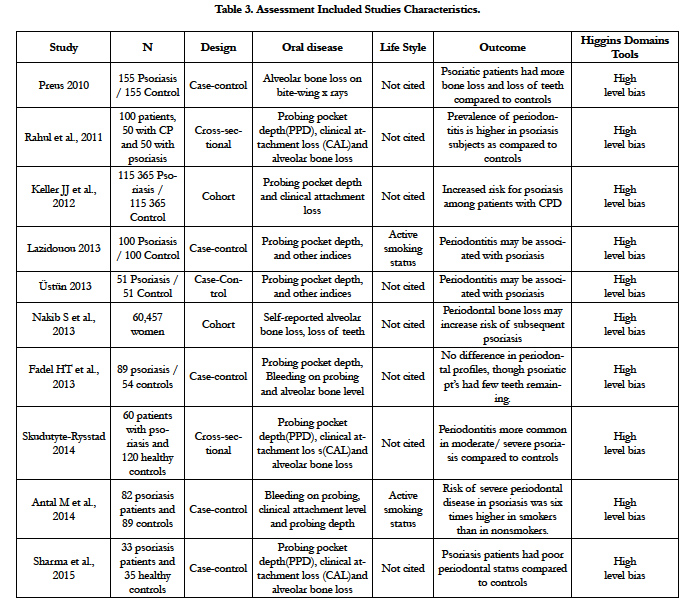
The assessment of methodological quality was done according the Consolidated Standards of Reporting Trials CONSORT statements [7], and was applied Appendix B Criteria Used in Quality Assessment of Non-Randomized Studies Scale [10]. Amstar Scale for systematic reviews and Higgins domains tools scale [9], were used for assessment of the methodological quality and for risk of bias, respectively (Table 3).
The study also was submitted to the register at International Prospective Register of Systematic Reviews PROSPERO, University of York/UK. The search included both randomized controlled trials and controlled trials that investigated dental improvement of psoriasis as the primary end point without limit for either date of publication or language.
We used the following databases as source up to 28 May 2015: Cochrane Skin Group Specialized Register; Cochrane Central Register of Controlled Trials (CENTRAL) 2014, Issue 5; MEDLINE from 1946 (via Pubmed); Embase from 1974; CINAHL (Cumulative Index to Nursing and Allied Health Literature) from 1981; Salford Database of Psoriasis trials; ISI Web of Science; HealthSTAR; LILACS (Literatura Latino-Americana e do Caribe em Ciências da Saúde); IBECS (Índice Bibliográfico Español en Ciencias de la Salud) and hand search at Unifesp Library. The search strategy for MEDLINE can be seen in Box 1.
We aimed to identify all relevant Randomized Clinical Trials and other studies regardless of language or publication status (published, unpublished, in press, or in progress). The studies retrieved were assessed for eligibility by reading the title and abstract. All eligible studies were read in full text in the evaluation for inclusion. Both the process of assessing eligibility for the inclusion of studies was performed by two independent evaluators regarding the validity, content and disagreements were consensus resolved. The extraction of data from selected studies was based on the standard Cochrane Collaboration data extraction forms modified to meet the needs of the present review.
Results
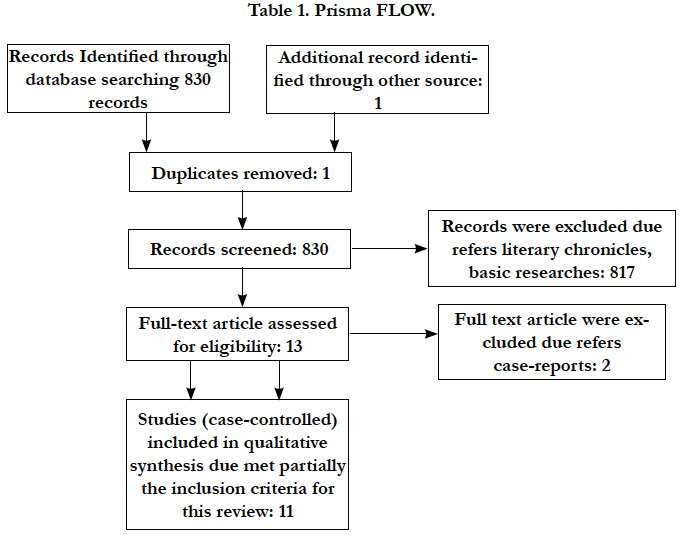
According to PRISMA statement the flow of information through the different phases of a systematic review concerning the identification, screening, eligibility and inclusion of studies was made [11] (Table 1).
There are different issues about oral health status related to the observed outcomes. None of included studies was able to answer PICO question completely. It was recovered 2085 studies, 11 were able to fill partially the inclusion criteria for this review despite 293.231 participants (Table 2).
The highest level of evidence obtained was 2 A (Randomized Clinical Trial), for the oral psoriatic arthritis forms at TMJ issue with one study. About the periodontal diseases issue was obtained the second highest level of evidence 2B (Clinical Trial Case-Control), with 10 studies. The concordance rate between evaluators was 98% [12]. Under these circumstances, and due the practical relevance of the theme, it was performed a qualitative analysis of included studies [10].
The strengths of the present study were in the broad literature search, explicit methods, selection and evaluation of duplicate studies, assessment of the methodological quality of the included studies, summaries of the findings. The retrieved studies varied widely in many aspects. The main limitations of present review were due to the lack of inclusion of unpublished studies and due to sparse studies with poor methodological quality which did not allow us to make any statistical analysis, nor measure of treatment effect, the impossibility to state any unit of analysis issues, nor subgroup analysis and investigation of heterogeneity either, even it was not available to consider the P value for each assessed study. Additional points; the limitations of the review were to show how to avoid these limitations, or make them less likely to influence the results. It was not possible to establish any comparison of results with the existing literature as this did not exist. However, it would be interesting to compare dental health studies in other systemic autoimmune diseases (lupus, for example) or chronic skin (vitiligo, for example) but also no such studies were found. In order to allow a comprehensive mapping of knowledge it were stated issues but at practices all these different clinical conditions often overlap resulting in the complex observed outcomes. No study had high enough methodological standards to be able to permit the establishment a cause-effect relationship between the conditions. About the issues, only one of those had 10 studies which was able to fill partially the inclusion criteria for this review. The methodological quality and risk of bias were assessed by APPENDIX B scale and Higgins domains tools, respectively.
Discussion
Psoriasis and oral health status can influence themselves reciprocally with relevance for observed outcomes.
It Means That Present Issues are the Oral Manifestations of Disease.
Although it is found different types of oral lesions related to psoriasis (whitish minimum round or oval zones, when scraped to leave the mucous surface bloody [17]); (surrounded whitish plaque reddened similar to psoriasis skin lesions [17]); (bright red mucosal lesions). Often they are in pustular psoriasis forms, erythrodermic psoriasis and Reiter's syndrome[17]as well.
Benign migration glossitis or geographic tongue (G.T.) and stomatitis are at a migration [17,36,51,52] (SAM), have more published studies and are psoriasis.
(G.T.) is one the most prevalent [89,95,97] and is an oral lesion that affects adults and children 98. It is a benign inflammatory disorder at the dorsum and margins of the tongue where occurs the replacement the filiform papilla epithelium to irregular central rythematous patches surrounded by elevated whitish band-like border. The lesions are able to change the location, size, and pattern over a period of time, and may spontaneously exhibit periods of remission and exacerbation, with generally good prognosis. (G.T.) sometimes causes pain and burning sensation and poor response to ordinary remedies 90.
Often (G.T.) and (S.A.M.) are clinical findings with few understanding, and there is not yet consensus between authors[121], about many aspects of pathogenesis, despite already is known that both lesions share histological similarities to psoriasis [17,66,67]. Under these circumstances (G.T.) and (S.A.M) is important to be stated these lesions are psoriasis, including, sometimes they can overlap with other genetic diseases and to be present in different clinical conditions89,95,98,125 and syndromes [85,86,88,92,94], where this oral psoriasis forms must to be faced as comorbidities17. In a time line, there are reports about (G.T. and S.A.M.) were induced by anti-cancer therapies when the drugs were used for the treatment of psoriasis systemic [75,76].
In other hand, there is a report about anti psoriatic therapy with etretinate that was observed a remarkable regression in the (G.T.) [75] suggesting the (G.T.) is a typical symptom of psoriasis pustulosa generalisata[75].
Reports about the existence of manifestation of psoriasis arthritis in the temporomandibular joint (TMJ) including imaging features, and TMJ anquilosis for both gender, in different ethnics, and ages[54,55,99,100, ,102, 104,105,106,107,108,109,110,111].
A cross-sectional survey randomly selected sample of 690 elderly people in Africa to assess oral mucosal lesions and temporomandibular joint impairment, and (22.46%) had one or more oral pathology lesions, representing infection-related swelling, noninfection- related swelling, pre-malignant lesions, denture stomatitis, non denture based ulcers, angular cheilitis, geographic tongue, scrotal tongue, lichen planus, hyper-pigmentation and TMJ impairment[101]. There are reports about well successful surgical approaches for the TMJ disorders in the psoriatic arthritis105, Although one recovered study stated to be randomized, for the issue psoriasis at TMJ, it was not possible assess completely the criteria high level of biases.
It means when the disease are able to induce oral manifestations and oral health status are able to lead psoriasis at different outcomes.
No study was able to state which disease came first. The scope
of present study was to assess the possibility that the periodontal disease can to contribute to inducing or worsening psoriasis lesions [126].
The global prevalence of dental diseases is high. Some between 4% to 12% adults in U.S.A. are affected with advanced periodontitis [58]. Evidence shows that psoriasis has multi factor origins, with the coexistence of underlying endogenous factors to its onset, in both, for triggering and for exacerbation, like skin trauma [46] (physical, pharmacological, electrical, surgical), successive upper respiratory tract infections[47], improper bowel, liver functions, inadequate nutrition, metabolic, immune disorders86 and periodontal diseases [6,43,44,48,49,50,78,83].
Emotional distress, is one of most important risk factor related to Psoriasis because affects the disease in many ways. The implications with periodontal diseases are because often psoriasis patient under negative emotional influences living including periods in which there is a decrease of personal care including proper oral hygiene[37]. It leads to the oral dysbiosis that made the Psoriasis worse.[126]
One study compared the prevalence of periodontitis and alveolar bone loss among individuals with skin forms psoriasis and a group of randomly selected controls showed the prevalence of moderate and severe periodontitis was significantly higher among psoriasis (24%) when compared to healthy controls (10%). Psoriasis had significantly more missing teeth and more areas with plaque and bleeding on probing, and 36% of psoriasis cases had one or more sites with radiographic bone loss ≥3 mm, compared to 13% of controls [44].
One study with 51 patients recommended that periodontal evaluation is important in psoriatic arthritis [6]. Including there is a study about non-plaque-related muco cutaneous disorders related to the chronic diseases, a special form of psoriasis in gums defined as desquamative gingivitis 91.
2.2.1. Inducing oral adverse outcomes: Drugs used to treat psoriasis conventionally are able to be related
to the systemic or oral adverse events [13,14], No reports about use of topical treatments for psoriasis inducing oral adverse events. There are reports about the use of systemic drugs e.g. retinoids, cyclosporine, vitamin D, and acitretine that are able to produce oral adverse events of light-to-mild severity, e.g., cheilitis, blisters, xerostomia, dental calculus, gingival hyperplasia, concussions, teeth loss or implants loss [4,15-20]
There is a report of mild to severe primary adverse events, related anti-EGFR-induced mucositis, BRAF-inhibitor-associated hyperkeratosis, osteonecrosis of the jaw observed, (G.T.) (S.A.M.) induced by angiogenesis inhibitors, and other oral lesions of psoriasis specifically linked with imatinib[75]. Report about mucositis due the use of other antineoplastic drugs (e.g. methotrexate)[16] and other substances as bevacizumab[14,76 ,but its impact on observed outcomes, and repercussions about costs or quality of life on psoriasis are still unclear [17,18,20,75,77].
2.2.2. Worsening oral adverse outcomes. There is reports about IgA deficiency and opportunistic infections such as herpes labialis, oral candidiasis, folliculitis induced anti-TNF-α treatment in a psoriatic arthritis patient. It also was observed previous local risk factors, such as (G.T.), (S.A.M.), (F.T.) and oral dysbiosis overlapped for observed outcomes.[79-81] In a time line, there are reports about (G.T. and S.A.M.) were induced by anti-cancer therapies when the drugs were used for the treatment of psoriasis systemic[75,76].
2.2.3. Inducing oral favorable outcomes. There is a case report about a 7-year-old female patient with generalized pustular psoriasis associated with (G.T.) and anti psoriatic therapy with etretinate that caused a remarkable regression in the tongue lesions77,suggesting the (G.T.) is a typical symptom of psoriasis pustulosa generalisata[75].
The scope is not to assess the impact of medications in Dentistry 130 but is relevant to consider the dental issues for drugs used in management of diseases related to the psoriasis, due the potential risk factors can stratify, resulting in adverse outcomes.
In a temporal perspective, it is possible to assess diseases related to psoriasis and its treatments, such as previous, simultaneous and subsequent clinical events to the appearance of skin lesions [17,28]
2.3.1. For Prior Morbidities related to the psoriasis.
There are many clinical conditions able to promotes Psoriasis, such hormonal changes[29] ,chronic inflammation[30] ,respiratorytract infections[31] ,intense emotional distress32,37 ,metabolic syndrome [26,33] ,hypertension[34], ,circulatory diseases35, recurrent skin infections[17] and mucous diseases36 and their treatments are among the triggers to the diseases that could be consider prior morbidities in psoriasis[38] because often are overlap with it. Many of these diseases require drugs (e.g. anticoagulants) that are able to produce oral cavity impairment[112] as bleeding. Also drugs used for management chronic illness also can overlap with dental procedures 22 and lead to oral adverse outcomes. Some times these events produce distress in many aspects able enough to result in a new case of Psoriasis as primary outcome [3,24,25]
2.3.2. For Co morbidities related to the psoriasis. Different uncontrolled clinical conditions can lead to psoriasis,
with simultaneous occurrence of the skin lesions[17]. Once the skin lesions, the more out of control are listed above clinical conditions, the greater the chances of occurrence of overlap syndrome. Among the more prevalent comorbidities, gums diseases[28] is one more prevalent and are also related with motivational level concerning self-care patient[126]. An important aspect is regarding mental health issues of psoriasis[32] , There are studies related to anxiety disorders occurrence simultaneous to skin lesions (e.g. phobias, panic, generalized anxiety, post-traumatic stress, acute anxiety, attention-deficit, hyperactivity) [37] and dental anxiety[39] as well. Many of these, often requires drugs for control (e.g., lithium) 27 used as co-intervention and able to generate skin unfavorable outcomes [39]. For the management of these problems, there are no pharmacological resources for psoriasis[37].
2.3.3. For Post Morbidities related to the psoriasis : Reports about post-morbidities psoriasis and its treatments demonstrated that can produce systemic problems and oral insults. Neural psychiatric disorders, (e.g. mood disorders)[39] alcoholism can lead to damage e.g. hepatitis, cirrhosis, changes in liver function40 and oral lesions as well, e.g. accumulation of dental plaque, oral dysbiosis and coated tongue, that lead to dental decay, periodontal diseases and tooth loss [33,36] The treatments for these diseases usually require many drugs, which can overlap and worsen oral health status, and aggravating psoriasis and even reducing life expectancy [2,4,24,27,33,36]
For instance, the administration and discontinuation of systemic corticosteroids may result in worsen of the skin condition, even lead to the development of a severe form of psoriasis e.g. generalized pustular psoriasis which can also be triggered by hypocalcemia, which also affects mouth [17]. Anticonvulsants that affect oral health and can worsen psoriasis[113].
2.4.1.Inducing systemic favourable outcomes to the psoriasis:
There are reports about improvement in skin forms of psoriasis after adequate dental treatments [22,46, 82,126] (Table 3).
2.4.2.Triggering the systemic psoriasis. There is oral dysfunctional interaction between psoriasis and some dental treatments or some substances for dental use36. There is a case report specific about dental infection as trigger factor in palmo-plantar psoriasis [5].It is known that many products used in dentistry, have the composition, chemicals that can lead to frames contact stomatitis, after use, even in people without skin diseases, which already can be considered as an additional risk factor, like acrylic restorations can worsen psoriasis [42].
2.4.3.Worsening the psoriasis : There are additional reports about important adverse events relating the worsening of psoriasis to local and systemic adverse outcomes such as dermatologic exacerbation after stressful dental procedures e.g. endodontics procedures, dental implant placement, or failures in dental care planning, that can act as a ‘trigger’ in psoriasis patients or about worsening outcomes e.g. some acrylic dental restorations [28,43] The level of evidence for oral implant therapy in patients with systemic conditions is very low[125].
There are many clinical conditions with the possibility to become oral risk for the universe of mouth diseases. The scope was to assess its impact only on oral issues when related to the exacerbationsof psoriasis.
At this scenario, some oral structures e.g. (F.T.), coated tongue (C.T.), teeth edges, dental decays can overlap with local environmental factors e.g. oral dysbiosis[36,53] and produce unfavorable outcomes.
(F.T.) also known as scrotal tongue, lingua plicata, plicated tongue and furrowed tongue is a benign condition, considered a slight variation of the normal pattern[17], related to genetic inheritance, is one of most frequent malformation of the tongue, where the deep crypts (fissures) are observed on the dorsum of the tongue. Although these grooves may look unsettling, the condition is usually painless. Some individuals may complain of an associated burning sensation[17, 81].
It is a relatively common condition, with a prevalence of between 6.8% to 11% found among children123,127. The prevalence of the condition increases significantly with age, occurring in 40% of the population after the age of 4028,. Thus, (F.T.) appears in different clinical conditions, and some syndromes, and it can stratify with other risks factors such as dysfunctional nutrition, inflammatory processes and mycosis that allows the worsening not only psoriasis[36,51,68,-71], but several other conditions that is one of the most prevalent sign, like in Melkersson-Rosenthal syndrome (along with facial nerve paralysis and granulomatous cheilitis). It is also seen in most patients with Down syndrome, in association with geographic tongue, in patients with oral manifestations of psoriasis, and in healthy individuals. Fissured tongue is also sometimes a feature of Cowden's syndrome73.
In some circumstances (F.T.) can overlap with oral dysbiosis resulting in halitosis, and so, it is important to give instructions to the patients for mechanical tongue cleansing in the oral hygiene routine. Since (F.T.) is entirely benign, no other treatment is indicated and the patient should be reassured that it is a common variance of the normal appearance of the tongue [81, 114, 115, 116,117]
(C.T.) coated or white tongue occurs when the surface is colonized by bacteria or fungi, and dead cells become trapped between the small structures on the tongue surface.
About tongue lesions a study in India with 4926 patients examined students for two 2 years, and demonstrated that its prevalence is about 12.07%, and among the most common lesion diagnosed was (C.T.) affecting 28.0% of the subjects, followed by (G.T.) 16.4%, (F.T.) 14.9% and hairy tongue (H.T.)11.5%. Males were more frequently affected than females. The most common systemic condition observed in the patients with tongue lesions was anaemia (189), followed by hypertension (47) and diabetes mellitus (38)[93, 97].
A study compared the prevalence of GT/FT and the correlation between tongue lesions and psoriasis severity using the PASI. 348 psoriasis and 348 healthy controls were selected. According to the age of psoriasis onset, the individuals were classified as having early psoriasis and late psoriasis. The severity of psoriasis was determined according to PASI. There is association between psoriasis intensity and GT and GT is associated with disease severity and may be a marker of the psoriasis severity [95].
The study of Dafar et al.120 identified an association between GT and anti-hypertensive medications, as well as the use of Swedish snus. Non-referred patients with GT (GTgp; n = 130) and FT (FTgp; n = 62) were examined by general practitioners (gp) and compared to a control group without oral mucosal lesions (C; n = 1029). Referred patients with GT (GTs; n = 166) and FT (FTs; n = 15) were examined by oral medicine specialists (s) and compared to GTgp and FTgp. Statistical analyses were performed using unpaired t-test or Fisher's exact test. A multiple logistic regression model was developed to control for age and gender as confounders.
GT group patients used more anti-hypertensive medications and Swedish snus (p < 0.01). The GTgp group consisted of older males (p < 0.001) compared to C. Compared to the GTgp group, the GTs group was younger, more likely to have symptomatic lesions (p < 0.0001) and comprised of more females. Among the groups examined, FT patients had the highest mean age.It also found differences in the activities and symptoms of the lesions between referred patients and their counterparts of controls who were seen in general dental practice; these parameters influenced the results when these conditions were taken into account.
The study of Al-Maweri et al., 122 about prevalence of Oral Mucosa Lesions (OML) in 310 elderly people in Yemen indicated the most frequently observed lesions were fissured tongue (34.2%), benign tumors (17.1%), hairy tongue (16.5%), and qat-induced white lesions (12.6%). Hairy tongue, qat-induced white lesions, and shammah keratosis were associated with men (P < 0.01, P < 0.05, and P < 0.05, respectively), whereas geographic tongue was associated with women (P < 0.05). The presence of one or more lesions was significantly associated with low education level (P < 0.05). Certain OMLs showed a significant association with smoking and qat chewing (P < 0.05). No association was found between the occurrence of OMLs and denture wearing (P > 0.05).
The study of Pavelic et al., 124 to identify genetic changes that may be the early hallmarks of epithelial cell over proliferation, was searched for p53 and nm23-H1 allelic deletions in oral benign epithelial lesions. In the study group were 25 benign epithelial lesions (lichen planus--17; leukoplakia--8; recurrent aphthous ulcers-- 2; one specimen diagnosed as benign migratory glossitis). Among 21 samples analysed for exon 4 (p53 gene) LOH, only 6 were informative, with no loss of either allele. OF 23 samples tested for LOH at intron 6 of p53 gene, 8 were informative, again with no presence of LOH. For nm23-H1 gene, the analysis was performed on a total of 24 cases. Of them, 16 were informative, however, none exhibited LOH at this locus. In conclusion, whereas the presence of gross gene alterations (LOH) would have been definitive evidence for the involvement of p53 and/or nm23 in the hyper proliferation process, the absence of LOH does not exclude the presence of either smaller mutations, altered regulation of normal gene, or dysfunction at the level of wild type protein. Alternatively, p53 and nm23-H1 may have no relation to oral lesion formation, and cannot presently be considered as an early step in benign, tissue transformation.
Conclusion
For clinicians, It is important consider oral implications for all psoriasis patients. Despite the detailed search performed, among 10 selected, no study had high enough methodological standards that could permit establishing a cause-effect relationship between these variables, but the possibility of an association between psoriasis and oral health is known [56]. It is known that dental diseases can influence the individual’s overall health, but in psoriasis its influence can generate complex, intricate, and uncertain outcomes [48,50,57].
Even not performing the analysis of the intrinsic mechanism of the phenomena, it is known that some factors, such as, the periodontal disease microbiota may aggravate or predispose to systemic diseases [59]. It is known that the lack of control of hygiene can lead into infection of the respiratory tract, especially in patients with some comorbidities, like diabetes which can result in cardiovascular diseases (CVD), frequent in psoriasis.[19,24,49,58,60,61]
Postmortem studies from cardiovascular diseases patients demonstrate the similarity among microbiota dental bio-films from periodontal disease and those found in the large arteries of atherosclerotic plaques, inclusive, a clinical condition highly prevalent in psoriasis [31,49,57,64].
There are reports about periodontitis and chronic plaque psoriasis [3,28,44,47,65,66 and other clinical conditions associated, including one study related the jaw bone loss in patients with moderate to severe psoriasis and periodontal disease is greater than the average bone loss in patient clinically similar conditions, but without periodontal disease.[43,126] According the results it is important that further studies should enhance the main aspects of methodological quality and randomized clinical trials with good quality of evidence need to be conducted to ensure that this scenario is adequately evaluated125. For the practice, regarding the diagnosis, treatment and control, will give better outcomes than we have be seen today [73].
The adoption of ASA physical status classification system from American Society of Anesthesiologists for assessment clinical risks should be adopted by professionals prior any dental interventions in psoriasis.[74,126] (Table 4).
Acknowledgements
Sincere thanks for their commitment and dedication to work with brilliant team of GEPSOS. Grupo de Estudos e Pesquisas Saúde Oral e Sistêmica Baseada em Evidências, Ambulatório Multidisciplinar Psoríase I Departamento Dermatologia, Escola Paulista de Medicina Unifesp in São Paulo, Brazil, which made possible the completion of the first training course in Psoriasis and Dental Care, held at the Fourtth National Meeting of Psoriasis 2016, Prof. Dr. Ana Baccari Kuhn, Prof. Dr. Denise Tiberio, Prof. Gilda Gionanonni, Aline Rocha, Ana Assumpción Vilés, Aparecida Monson, Aurora Gonçalvez, Debora Damasceno, Edson Brasil, Maria Beneticta Martins, Maria Christina Rodrigues, Miriam Goldstajn, Monica Okuraha, Nicezar Castro, Pedro Horvath, Renata Savino, Erika Justo (Universidade de São Paulo), Denise Cirino (Universidade de São Paulo), Cochrane Skin Group Not-tingham University / United Kingdom.
References
- Chen G, Zhou D, Zhang Z, Kan M, Zhang D, Hu X, et al., (2012) Genetic variants in IFIH1 play opposite roles in the pathogenesis of psoriasis and chronic periodontitis. Int J Immunogenet. 39(2): 137-43.
- Guinot C, Latreille J, Perussel M, Dosse N, Dubertret L, et al., (2009) Psoriasis: characterization of six different clinical phenotypes. Experimental Dermatology. 18 (8): 712–719.
- Parisi R, Symmons D, Griffiths C (2013) Global Epidemiology of Psoriasis: A Systematic Review of Incidence and Prevalence. J Invest Dermatol. (133): 377-385.
- Raychaudhuri S, Farber E (2001) The prevalence of psoriasis in the world. JEADV. 15(1): 16-17.
- Kikuchi N, Yamamoto T (2013) Dental infection as a triggering factor in palmoplantar pustulosis. Acta Derm Venereol. 93(6): 721-722.
- Üstün K, Sezer U, Kisacik B, Senyurt SZ, Özdemir E, et al., (2013) Periodontal disease in patients with psoriatic arthritis. Inflammation. 36(3): 665-659.
- Schulz K, Altman D, Moher D, Consort group (2010) CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. Ann Int Med.152(11): 726-732.
- Chan A, Altman D (2005) Identifying outcome reporting bias in randomised trials on PubMed: review of publications and survey of authors. BMJ. 330(7494): 753.
- Higgins J, Green S (editors) (2011) Cochrane Handbook for Systematic Reviews of Interventions 5.1.0. Qualitative research and Cochrane reviews. Cochrane Collaboration, Medical Research Council, United Kingdom.
- Bloomfield H, Greer N, Newman D, MacDonald R, Carlyle M, et al., (2012) Predictors and Consequences of Severe Hypoglycemia in Adults with Diabetes – A Systematic Review of the Evidence. Evidence-based Synthesis program.
- Moher D, Liberati A, Tetzlaff J, Altman D, The PRISMA Group (2009) Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Ann Intern Med. 3(3): 123-130.
- Fleiss JL (1981) Statistical methods for rates and proportions. (2nd Edn), Wiley : New York.
- Cunha Filho R, Almeida Júnior H, Breunig J (2011) Angiodema due to oral acitretin and isotretinoin. An Bras Dermatol. 86(4): 28-30.
- Popa A, Valla K, Radhakrishnan L, Cuellar S, Villano J (2012) Bevacizumab -induced oral mucositis in background of cutaneous plaque-type psoriasis. Ann Pharmac other. 46(11): e32.
- Di Giovanna J, Peck G (1987) Retinoid toxicity. Porg. Dermatol. 21: 1-8.
- Boffa M, Chalmers R (1996) Methotrexate for psoriasis. Clin Exp Dermatol. 21(6): 399-408.
- Camisa C (2003) Conditions associated with Psoriasis. Handbook of Psoriasis. (2nd Edn), Blackwell Science Inc: New York.
- Deeming G, Collingwood J, Pemberton M (2005) Methotrexate and oral ulceration. Br Dent J. 198 (2): 83-85.
- Cribier J (2006) Psoriasis under the microscope. JEADV. 20(s2): 3-9.
- Woolf R, West S, Arenas-Hernandez M, Hare N, Peters van Ton AM, et al., (2012) Methotrexate polyglutamates as a marker of patient compliance and clinical response in psoriasis: a single-centre prospective study. Br J Dermatol.167(1): 165-173.
- Laws P, Young H (2012) Current and emerging systemic treatment strategies for psoriasis. Drugs. 72(14):1867-1880.
- Basavaraj KH, Ashok NM, Rashmi R, Praveen TK (2010) The role of drugs in the induction and/or exacerbation of psoriasis. Int J Dermatol. 49(12):1351-61.
- Boehncke WH, Boehncke S, Tobin AM, Kirby B (2011) The ‘‘psoriatic march’’: a concept of how severe psoriasis may drive cardiovascular comorbidity. Exp Dermatol. 20: 303–307.
- Yussuf S, Reddy S, Ounpuu S, Anand S (2001) Global burden of cardiovascular disease. Part II: variations in cardiovascular disease by specific ethnic groups and geographical regions and prevention strategies. Circulation.104(23): 2855-2864.
- Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, et al., (2006) Risk of Myocardial Infarction in Patients with Psoriasis. JAMA. 296(14): 1735 -41.
- Pischon N, Heng N, Bernimoulin JP, Kleber BM, Willich SN, et al., (2007) Obesity, inflammation and periodontal disease. J Dent Res. 86: 400-409.
- Jafferany M (2008) Lithium and psoriasis: What primary care and family physicians should know? Prim Care Companion. J Clin Psychiatry. 10(6):435-439.
- Lazadiou E, Tsikrikoni A, Fotiadou C, Vakirlis E, Apalla Z, et al., (2013) Association of chronic plaque psoriasis and severe periodontitis: a hospital based case-control study. JEADV. 27: 967-972.
- Li WQ, Han JL, Manson JE, Rimm EB, Curhan GC, et al., (2012) Psoriasis and risk of nonfatal cardiovascular disease in U.S. women: a cohort study. Br J Dermatol. 166: 811-18.
- Brauchli YB, Jick SS, Miret M, Meier CR (2009) Psoriasis and risk of incident myocardial infarction, stroke or transient ischaemic attack: an inception cohort study with a nested case–control analysis. Br J Dermatol. 160:1048-56.
- Ahlehoff O, Gislason GH, Charlot M, Olesen JB, Skov L, et al., (2011) Psoriasis is associated with clin.cally significant cardiovascular risk: a Danish nationwide cohort study. J Intern Med. (270): 147-57.
- Gaston l, Crombez JC, Lassonde M, Bernier-Buzzanga J, Hodgins S (1991) Psychological stress and psoriasis: experimental and prospective correlational studies. Acta Derm Venereol Suppl (Stockh). 156 : 37-43.
- Alberti KG, Eckel RH, Grundy SM, Zimmet pz, Cleeman JI, et al., (2009) Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention;National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 120(16):1640-1645.
- Miller IM, Ellervik C, Yazdanyar S, Jemec GB (2013) Meta-analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol. 69(6):1014-24.
- Pietrzak A, Bartosińska J, Chodorowska G, Szepietowski JC, Paluszkiewicz P, et al., (2013) Cardiovascular aspects of psoriasis: an updated review. Int J Dermatol. 52(2): 153-162.
- James W, Berger T, Elston D (2011) Andrews’ Diseases of the Skin. Clinical Dermatology. (11th Edn), W.B. Saunders, US.
- Monson CA, Silva V, Andriolo RB, Kozasa EH, Sabbag CY, et al., (2014) Complementary therapies for chronic plaque psoriasis. Cochrane Database Syst Rev. 7: CD011243.
- Farber E, Nall L (1933) Psoriasis: a stress related disease. Cutis. 51(5): 332- 336.
- Alex Rubino, Alberto Sonnino, Bianca Pezzarossa, Nicola Ciani, Roberto Bassi (1995) Personality disorders and psychiatric symptoms in psoriasis. Psychological Reports. 77(1): 547-553.
- Higgins E (2000) Alcohol, smoking and psoriasis. Clin Exp Dermatal. 25(2): 107-110.
- Gage T, Pickett F (1997) Dental Drug Reference. St. Louis: Mosby Inc.; 1997. 2nd ed.
- Kosugi M, Ishihara K, Okuda K (2003) Implication of responses to bacterial heat shock proteins, chronic microbial infections, and dental metal allergy in patients with pustulosis palmaris et plantaris. Bull Tokyo Dent Coll. 44(3): 149-158.
- Skudutyte-Rysstad R, Slevolden EM, Hansen BF, Sandvik L, Preus HR (2014) Association between moderate to severe psoriasis and periodontitis in a Scandinavian population. BMC Oral Health. 14:139.
- Ishihara K, Ando T, Kosugi M, Kato T, Morimoto M, et al., (2000) Relationships between the onset of pustulosis palmaris et plantaris, periodontitis and bacterial heat shock proteins.Oral Microbiol Immunol. 15(4): 232-237.
- Murai O, Sasaki D, Ando Y, Fujimura A, Oikawa H, et al., (2012) Improvement of pustulosis palmaris et plantaris by periodontal infection control in a patient with chronic periodontitis. Clin Lab. 58(3-4): 323-327.
- Fry L, Baker BS, Powles AV, Fahlen A, Engstrand L (2013) Is chronic plaque psoriasis triggered by microbiota in the skin? Br J Dermatol. 169(1): 47-52.
- Antal M, Braunitzer G, Mattheos N, Gyulai R, Nagy K (2014) Smoking as a permissive factor of periodontal disease in psoriasis. PLoS One. 9(3): e92333.
- Preus H, Khanifam P, Kolltveit K, Mørk C, Gjermo P (2010) Periodontitis in psoriasis patients. A blinded, case-controlled study. Acta Odontol Scandinav. 68(3): 165-170.
- Keller JJ, Lin HC (2012) The effects of chronic periodontitis and its treatment on the subsequent risk of psoriasis. Br J Dermatol. 167(6): 1338-44.
- Fadel HT, Flytström I, Calander AM, Bergbrant IM, Heijl L, et al., (2013) Profiles of dental caries and periodontal disease in individuals with or without psoriasis. J Periodontol. 84(4): 477-85.
- Dreyer LN, Brown GC (2012) Oral manifestations of psoriasis. Clinical presentation and management. NY State Dent J. 78(3):14-18.
- Germi L, De Giorgi V, Bergamo F, Niccoli MC, Kokelj, et al., (2012) Psoriasis and oral lesions: multicentric study of Oral Mucosa Diseases Italian Group (GIPMO). Dermatol Online J.18(1): 11.
- Tomb R, Hajj H, Nehme E (2012) Oral lesions in psoriasis. Ann Dermatol Venereol. 137(11): 695-702.
- Vieira A, Rabelo L, Serra R (2009) TMJ ankylosis in children: aspects of surgical interest. Rev Cir Traumatol Buco-maxilo-fac. 9(1): 15-24.
- Oliveira JX, Brasil SA, Santos KCP (2011) Manifestation of psoriatic arthritis in the temporomandibular joint - imaging features. RFO. 16(1): 10-113.
- Yamada J, Amar S, Petrungaro P (1992) Psoriasis-associated periodontitis: a case report. J Periodontol. 63(10): 854-759.
- Higashi Y, Goto C, Jitsuiki D, Umemura T, Nishioka K, et al., (2008) Periodontal infection is associated with endothelial dysfunction in healthy subjects and hypertensive patients. Hypertension. 51(2): 446-453. https://www.ncbi.nlm.nih.gov/pubmed/18039979
- NHANES-National Health and Nutrition Examination Survey. Centers for Disease Control and Prevention , 2015.
- Hashmi S, Zeng QT (2006) Role of interleukin-17 and interleukin-17-induced cytokines interleukin-6 and interleukin-8 in unstable coronary artery disease. Coron Artery Dis. 17(8): 699–706.
- Tholen S, Lubach D (1987) Changes in the oral mucosa in psoriasis pustulosa generalisata. Hautarzt. 38(7): 419-421.
- Casper U, Seiffert K, Dippel E, Zouboulis C (1998) Exfoliatio areata linguae et mucosae oris: Eine Schleimhautmanifestation der Psoriasis pustulosa? Hautarzt. 49(11): 850-854.
- Desvarieux M, Demmer R, Rundek T, Bode-Albala B, Jacobs D, et al., (2003) Relationship between periodontal disease, tooth loss, and carotid artery plaque: the oral infections and vascular disease epidemiology study.Stroke. 34(9): 2120-2125.
- Khalili J (2008) Periodontal disease: an overview for medical practitioners. Likars’ka Sprava. (3-4):10-21.
- Akazawa H, Nishimura F, Maeda H, Takashiba S, Mine A, et al., (2006) Regression of pustulosis palmaris et plantaris by periodontal treatment in a subject with severe periodontitis. Int J Dermatol. 45(12): 1420-1422.
- Sharma A, Raman A, pradeep A (2014) Association of chronic periodontitis and psoriasis: periodontal status with severity of psoriasis. Oral Dis. 21(3): 314-319.
- Daneshpazhooh M, Moslehi H, Akhyani M, Etesami M (2004) Tongue lesions in psoriasis: a controlled study. BMC Dermatol. 4(1): 16.
- Kraether Neto L, Borba M, Figueiredo M, Cherubini K, Yurgel L (2004) Geographic tongue and psoriasis relationship. Rev Bras Patol Oral. 3(1): 32-35.
- Zargari O (2006) The prevalence and significance of fissured tongue and geographical tongue in psoriatic patients. Clin and Exper Dermatol. 31(2): 192-195.
- Koay CL, Lim JA, Siar CH (2011) The prevalence of tongue lesions in Malaysian dental outpatients from the Klang Valley area. Oral Dis. 17(2): 210-216.
- Silverman S, Eversole L, Truelove E (2002) Soft tissue disorders. In: Essential of Oral Medicine. (2nd Edn), Decker: Hamilton, Canada.
- Rioboo R, Pozo P, García R (2005) Epidemiología de la patología de la mucosa oral más frecuente en niños. Med Oral Patol Oral Cir Bucal. 10(5):376-387.
- Darwazeh A, Al-Aboosi M, Bedair A (2012) Prevalence of oral mucosal lesions in psoriatic patients: A controlled study. J Clin Exp Dent. 4(5): e286- 91.
- American Dental Association (1991) Specialty Definitions. www.ada.org
- American Society of Anesthesiologists (1995) Standards and Guidelines. www.asahq.org
- Sibaud V, Boralevi F, Vigarios E, Fricain J (2014) Oral toxicity of targeted anticancer therapies. Ann Dermatolo Vénéréol. 141(5): 354-363.
- Gavrilovic IT, Balagula Y, Rosen AC , Ramaswamy V, Dickler MN, et al., (2012) Characteristics of oral mucosal events related to bevacizumab treatment. oncologist. 17(2): 274-278.
- Younai FS, Phelan JA (1997) Oral mucositis with features of psoriasis: report of a case and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 84(1): 61-67.
- Oh TJ, Eber R, Wang HL (2002) Periodontal diseases in the child and adolescent. J Clin Periodontol. 29(5): 400-410.
- Iervolino, S Lofrano, M and Di Minno, M N D and Foglia, F and Scarpa, R and Peluso, R. Analisi computerizzata su alcuni fattori condizionanti la prevalenza e la distribuzione dell'artrite reumatoide. REUMATISMO.2012. 64; 1
- Corazza M, Zauli S, Ricci M, Borghi A, Rossi R, et al., (2013) Does antitumour necrosis factor-alpha increase oral candida colonization? A casecontrol study in psoriatic patients. Acta Derm Venereol. 93(3): 352-353.
- Dudko A, Kurnatowska A, Kurnatowski P (2013) Prevalence of fungi in cases of geographical and fissured tongue. Ann parasitol. 59(3): 113-117.
- Delaney JE (1995) Periodontal and soft-tissue abnormalities. Dent Clin North Am. 39(4): 837-850.
- Ganzetti G, Santarelli A, Pozzi A, Molinelli V, Minnetti A, et al., (2014) Periodontal disease: an oral manifestation of psoriasis or an occasional finding? Drug Dev Res. 75(1): S46-49.
- Mayer Y, Rina A, Moscovici Y, Machtei E (2013) Periodontal condition of patients with autoimmune diseases and the effect of anti-tumor necrosis factor-α therapy. J Periodontol. 84(2): 136-142.
- Beiraghi S, Myers S, Baker S (2007) Oral manifestations of a possible new periodic fever syndrome. Pediatr Dent. 29(4): 323-326.
- Bassim G, Domingo P, Balog P, Guadagnini B, Gahl W, et al., (2010) Craniofacial and dental findings in cystinosis. Oral Dis. 16(5): 488-495.
- Afanas V, Muromtsev A, Derkach N (2008) [Salivary glands and oral mucous membrane status in patients with chronic hepatitis]. Stomatologii͡a. 87(2): 31-33.
- Cerqueira D, Ferraz I, Pomarico R (2008) Orofacial manifestations of Robinow's syndrome: a case report in a pediatric patient. Oral surg oral med oral pathol oral radiol endod. 105(3): 353-357.
- Bernitz H, Ligthelm A (1998) Prevalence of oral pathoses in a private dental practice: a 30 month survey. SADJ : journal of the South African Dental Association = tydskrif van die Suid-Afrikaanse Tandheelkundige Vereniging.53(12): 531-534.
- Flaitz C, Baker K (2000) Treatment approaches to common symptomatic oral lesions in children. Dental Clinics Of North America. 44(3): 671-696.
- Aldahish F, Almotawa Z, Kujan O (2015) The Differential Diagnosis of Desquamative Gingivitis: Review of the Literature and Clinical Guide for Dental Undergraduates. J Int Oral Health. 7(1): 88–92.
- Bassim C, Gautam P, Balog P, Guadagnini J, Gahl W , et al., (2010) Craniofacial and dental findings in cystinosis. Oral Diseases. 16(5): 488-495.
- Järvinen J, Mikkonen J, Kullaa A (2014) Fissured tongue: a sign of tongue edema? Med Hypotheses. 82(6): 709-712.
- Cerqueira F, Souza R (2008) Orofacial manifestations of Robinow's syndrome: a case report in a pediatric patient. Oral surg oral medi oral pathol oral radiol endod. 105(3): 353-357.
- Gonzaga H, Jorge A, Tomimori J, Barbosa C (2008) Study of incidence and heritability of psoriasis and benign migratory glossitis in a population of the State of São Paulo, Brazil. J Eur Acad Dermatol Venereol. 24(1): 12-14.
- Patil S, Kaswan S, Rahman F, Doni B (2013) Prevalence of tongue lesions in the Indian population. J clin exp dent. 5(3): .e128-e132.
- Picciani B, Santos T, Domingos C, Carneiro T, Avelleira S, et al., (2015) Geographic tongue and fissured tongue in 348 patients with psoriasis: correlation with disease severity. Scientific World Journal. 2015: 564326.
- Flaitz C, Baker K (2000) Treatment approaches to common symptomatic oral lesions in children. Dental Clinics Of North America. 44(3): 671-696.
- Aviel Y, Butbul T, Schneider S, Dhillon B, Feldman R, et al., (2011) Juvenile Psoriatic Arthritis: Juvenile arthritis with psoriasis? Pediatr. Rheumatol. 11: 11.
- Farronato G, Garagiola U, Vera P, Bellintani C (2010) Psoriatic arthritis: temporomandibular joint involvement as the first articular phenomenon.Quintessence int. 41(5): 395-398.
- Taiwo J, Kolude O, Bamidele V (2009) Oral mucosal lesions and temporomandibular joint impairment of elderly people in the South East Local Government Area of Ibadan. Gerodontology. 26(3): 219-224.
- Mattila M, Könönen M, Mattila K (1995) Vertical asymmetry of the mandibular ramus and condylar heights measured with a new method from dental panoramic radiographs in patients with psoriatic arthritis. J Oral Rehabil. 22(10): 741-745.
- Bjørnland T, Larheim T (1995) Synovectomy and diskectomy of the temporomandibular joint in patients with chronic arthritic disease compared with diskectomies in patients with internal derangement. A 3-year followup study. Eur J Oral Sci. 103(1): 2-7.
- Könönen M, Wolf J, Kilpinen E, Melartin E (1995) Radiographic signs in the temporomandibular and hand joints in patients with psoriatic arthritis.Acta Odontol Scand. 49(4): 191-196.
- Könönen M, Kilpinen E (1990) Comparison of three radiographic methods in screening of temporomandibular joint involvement in patients with psoriatic arthritis. Acta Odontol Scand. 48(4): 271-277.
- Wenneberg B, Könönen M, Kallenberg A (1990) Radiographic changes in the temporomandibular joint of patients with rheumatoid arthritis, psoriatic, arthritis, and ankylosing spondylitis. J craniomandib disord. 4(1): 35-39.
- Könönen M (1987) Clinical signs of craniomandibular disorders patients with psoriatic arthritis. Scand j dent res. 95(4): 340-346.
- Könönen M, Ekholm P, Mäkilä E (1987) Craniomandibular disorders in elderly with psoriasis. Compr gerontol A. 1(1): 25-28.
- Könönen M (1987) Craniomandibular disorders in psoriatic arthritis. A radiographic and clinical study. Proc Finn Dent Soc. 83(8-10): 1-45.
- Avrahami E, Garti A, Weiss-Peretz J, Yaron M (1986) Computerized tomographic findings in the temporomandibular joint in patients with psoriatic arthritis. J Rheumatol. 13(6): 1096-1098.
- Iacopino AM, Wathen WF (1993) Craniomandibular disorders in the geriatric patient. J Orofac Pain. 7(1): 38-53.
- Connolly S, Poque J, Hart R, Pfeffer M, Yusuf S, et al., (2006) Clopidogrel plus aspirin versus oral anticoagulation for atrial fibrillation in the Atrial fibrillation Clopidogrel Trial with Irbesartan for prevention of Vascular Events (ACTIVE W): a randomised controlled trial. Lancet. 367: 1903–12.
- Cornacchio A, Burneo J, Aragon C (2011) The Effects of Antiepileptic Drugs on Oral Health. J Can Dent Assoc. 71: b140.
- Daneshpazhooh M, Nazemi TM, Mohammad-Java, Bigdeloo L, Yoosefi M (2007) Mucocutaneous findings in 100 children with Down syndrome. Pediatr Dermatol. 24(3): 317-320.
- Bilgili SG, Akdeniz N, Karadag AS, Akbayram S, Calka O, et al., (2011) Mucocutaneous disorders in children with down syndrome: case-controlled study. Genet Couns. 22(4): 385-392.
- Petrini M, Costacurta M, Ferrante M, Trentini P, Docimo R, et al., (2014) Association between the organoleptic scores, oral condition and salivary β-galactosidases in children affected by halitosis. Int j dent hyg. 12(3):2131- 218.
- Reamy BV, Derby Richard, Bunt Christopher W (2010) Common tongue conditions in primary care. Am fam physician. 81(5): 627 – 634.
- Gerressen M, Ghassemi A, Stockbrink G, Riediger D, Zadeh MD (2005) Melkersson-Rosenthal syndrome: case report of a 30-year misdiagnosis. J Oral Maxillofac Surg. 63(7): 1035-1039.
- Du ZF, Xu CM, Zhao Y, Liu WT, Chen XL, et al., (2012) Two novel de novo mutations of KRT6A and KRT16 genes in two Chinese pachyonychia congenita pedigrees with fissured tongue or diffuse plantar keratoderma.Eur j dermatol. 22(4): 476-480.
- Dafar Amal, Çevik-Aras Hülya, Robledo-Sierra Jairo, Mattsson Ulf, Jontell Mats (2016) Factors associated with geographic tongue and fissured tongue. Acta odontologica Scand. 74(3): 2010-216.
- Rezaei F, Safarzadeh M, Mozafari H, Tavakoli P (2014) Prevalence of Geographic tongue and Related Predisposing Factors in 7-18 Year-Old Students in Kermanshah, Iran 2014. Glob J Health Sci. 7(5): 91-95.
- Al-Maweri Sadeq Ali, Al-Jamaei Aisha Ahmed, Al-Sufyani Ghadah A, Tarakji Bassel, Shugaa-Addin Bassam (2015) Oral mucosal lesions in elderly dental patients in Sana'a, Yemen. J Int Soc Prev Community Dent. 5(1):S12-S19.
- Huamei Y, Yu Z, Xin Z, Ga L, Qianming C (2015) Research progress on the risk factors of geographic tongue. Hua xi kou qiang yi xue za zhi. 33(1): 93-97.
- Pavelic J, Gall-Troselj K, Mravak-Stipetic M, Pavelic K (1998) The p53 and nm23-H1 genes are not deleted in oral benign epithelial lesions. Anticancer Res. 18(5): 3527-31.
- Monson C, Porfírio G, Riera R, Tweed J, Petri V, et al., (2016) Periodontal Aspects for Psoriasis: A Systematic Review. Clin Res Dermatol Open Acess. 3 (1): 1-8.
- Ciancio S (2004) Medications’ impact on oral health. JADA. 135(10): 1440-1448.