Crocin Ameliorated Atopic Dermatitis Induced in Mice by Inhibiting E-Cadherin/AKT Pathway
Alyoussef A*
Associate Professor of Dermatology & Venerology (Hair AND Scalp Disorders), Department of Internal Medicine (Dermatology), Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia.
*Corresponding Author
Abdullah Alyoussef MD, DES,
Associate Professor of Dermatology & Venerology (Hair AND Scalp Disorders),
Department of Internal Medicine (Dermatology), Faculty of Medicine, University of Tabuk, Tabuk, Saudi Arabia.
Tel: +966-50-4629800
E-mail: aalyoussef@ut.edu.sa
Received: November 21, 2019; Accepted : December 31, 2019; Published: January 04, 2020
Citation: Alyoussef A. Crocin Ameliorated Atopic Dermatitis Induced in Mice by Inhibiting E-Cadherin/AKT Pathway. Int J Clin Dermatol Res. 2020;8(1):248-252. doi: dx.doi.org/10.19070/2332-2977-2000054
Copyright: Abdullah Alyoussef© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Atopic dermatitis (AD) is a chronic relapsing inflammatory disease characterized by inflammatory cell infiltration in skin.
Objectives: To evaluate protective effects of crocin in AD on E-cadherin/AKT pathway.
Methods: AD was induced by repeated application of 2,4-dinitrochlorobenzene (DNCB) on ears and shaved dorsal skin of mice. After development of AD, part of mice were treated with 20mg/kg crocin. Part of skin was isolated for assessment of E-cadherin, AKT, MMP9 and syndecan-1.
Results: Investigation of histological sections revealed that crocin ameliorated AD-induced skin tissue damage and reduced acanthosis of epidermis and infiltration with mast cells. Crocin attenuated AD-induced deactivation of E-cadherin and reduced gene and protein expression of AKT, MMP9 and syndecan-1.
Conclusions: Crocin alleviated AD induced in mice activation of E-cadherin leading to inhibition of inflammatory cell migration.
2.Background
3.Materials and Methods
3.1 Animals
3.2 Animal sacrifice and collection of samples
3.3 Morphologic analysis of skin tissue
3.4 ELISA determination
3.5 Quantitative real-time polymerase chain reaction (RT-PCR)
3.6 Statistical analysis
4.Results and Discussion
5.Conclusion
6.References
Keywords
E-Cadherin; Matrix Metalloproteinase 9 (MMP9); Protein Kinase B (PKP, AKT); Syndecan-1.
Background
Atopic Dermatitis (AD) is heritable disease that involves multiple genes in its induction and progression. It is widely admitted that AD is a systemic disease with both atopic and nonatopic comorbidities [1, 2]. However, AD may occur at any age, AD starts during the first two years of life [3]. AD is characterized by erythema, edema, lichenification, excoriations, oozing and crusting. AD mainly occurs on ankles, wrists, face, hands and feet, neck and upper chest [4]. Pruritus is a pivotal feature of AD and bring out comorbidities such as psychological distress, sleep loss and increased healthcare utilization. It may be accompanied with symptoms of bronchial asthma, nasal mucosa inflammation and conjunctivitis [5]. Severe cases of AD is correlated with poorer overall health as it causes many disorders such as impaired sleep and increased healthcare utilization [6].
Patients with AD are characterized by skin barrier dysfunction leading to penetration of allergens into the skin, associated with an increased cutaneous inflammation [7]. Many genetics factors might spoil diverse aspects of barrier function. In addition, environmental exposures may affect the barrier function such as soaps, detergents, exogenous proteases and repetitive scratching [8].
Crocin is the major component of Gardenia jasminoides Family Rubiaceae. Crocin was proved to treat AD via inhibition of histamine [9] and blocking of ERK-MAPK/NFκB/STAT1 [10]. Therefore, the goal of this study was to determine the effect of crocin on E-cadherin/AKT signaling pathways in atopic dermatitis.
The local ethical committee approved the animal protocol. Fourweek-old BALB/c mice were housed under pathogen-free conditions with normal 12-h light-dark cycle. Mice were distributed into four groups with ten mice each:
Hair of the back of mice was shaved and rubbed with mixture of acetone: olive oil (3:1) was as well as the back of both ears of mice.
Mice were given 20 mg/kg crocin (Sigma Aldrich Chemicals Co., St Louise, MO, USA) subcutaneously three times per week for three weeks.
After 24 hours of removal of the dorsal hair, 100 μl of 1% 2,4-dinitrochlorobenzene (DNCB) solution in acetone : olive oil (3:1) was utilized on back skin and back of both ears for sensitization. After five days, 150 μl of 0.2% DNCB was applied on the skin of the back and behind both ears three times a week for three weeks [11, 12].
After induction of AD, mice were treated with 20 mg/kg of crocin subcutaneously three times per week for another three weeks.
Animals were sacrificed by decapitation. Skin samples were removed and stored at -80°C.
Five-micrometer thickness sections were cut from paraffin blocks, stained with toluidine blue and inspected in a blind manner using Nikon Digital Camera (Japan).
Commercially available ELISA kits was used for determination of skin levels of E-cadherin, AKT, syndecan-1 (Thermo Fisher Scientific Inc., Waltham, MA, USA) and MMP9 (R&D Systems, Inc. Minneapolis, MN, USA) following the manufacture protocol.
RNeasy Mini kit (Qiagen, USA) was used for separation of total RNA. Next, Maxima® SYBR Green/Fluorescein qPCR Master Mix (Fermentas, USA) was used for estimation of total amount of RNA followed by reverse-transcription of 1μ RNA into cDNA by QuantiTect® Reverse Transcription Kit (Qiagen, USA). AKT, MMP9 and syndecan-1 mRNA levels were determined by Maxima® SYBR Green/Fluorescein qPCR Master Mix by Rotor- Gene Q (Qiagen, USA). Meanwhile, GAPDH was implemented as an internal reference. The specific PCR primers (Table 1) were prepared by the help of Primer Express 3.0 (Applied Biosystems, USA).
The mean ± standard error was used. Sample normality were checked by Kolmogorov-Smirnovtest was used. Comparison between groups was evaluated by ANOVA followed by post hoc Bonferroni correction tests. SPSS version 20 was used for statistical computations. Statistical significance was predefined as P ≤ 0.05.
Results and Discussion
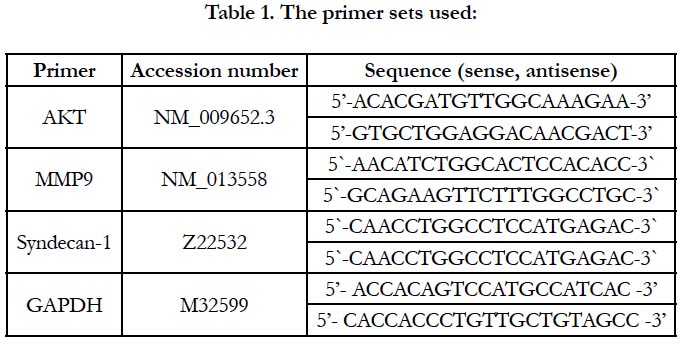
Atopic dermatitis resulted in 61% reduction in the skin level of E-cadherin when compared with the control group. Treatment of AD mice with crocin resulted in about 2-fold elevation in E-cadherin as compared with AD mice but still significantly less than the control group. Crocin did not affect the E-cadherin of control group (Figure 1). E-cadherin is a transmembrane protein and one of the tight junction [13]. The lesion of AD in humans is associated with down-regulation of E-cadherin [14]. In the inflamed skin lesion of AD patients, off note, E-cadherin expression enhances the induction of T regulatory cells in mice [15]. Crocin was reported to reduce the ability of cells invasion, migration and adhesion by increasing the expression E-cadherin in melanoma metastatic model in mice [16] and prostate cancer cell lines [17]. Up to our knowledge, this is the first time to examine the effect of crocin on E-cadherin in atopic dermatitis model in mice.
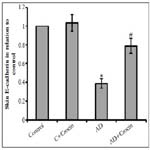
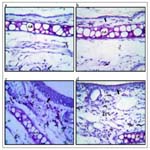
Histological observations of skin section of AD mice stained with toluidine blue declared the presence of acanthosis of epidermis (broad arrows) and infiltration with mast cells (thin arrows). All these effects were attenuated by crocin (Figure 2).
Figure 1. Effect of atopic dermatitis (AD) alone and in combination with 20 mg/kg crocin on skin levels of E-cadherin. *: significant difference compared with the control groups at p<0.05. #: significant difference compared with atopic dermatitis group at p<0.05.
Figure 2. Effect of atopic dermatitis (AD) alone and in combination with 20 mg/kg crocin on mice skin sections from control (a), treated control (b), atopic dermatitis (c) and treated atopic dermatitis (d) mice, stained with toluidine blue. Sections showing acathosis of the epidermis of the skin (broad arrows) and infiltration of mast cells (thin arrows).
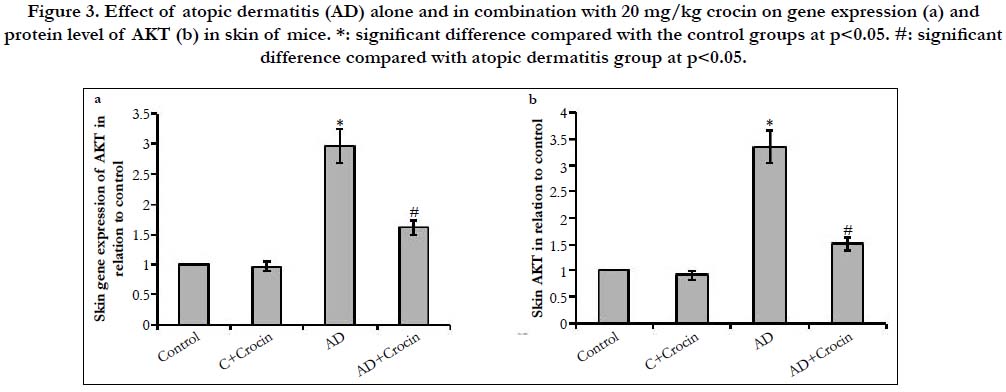
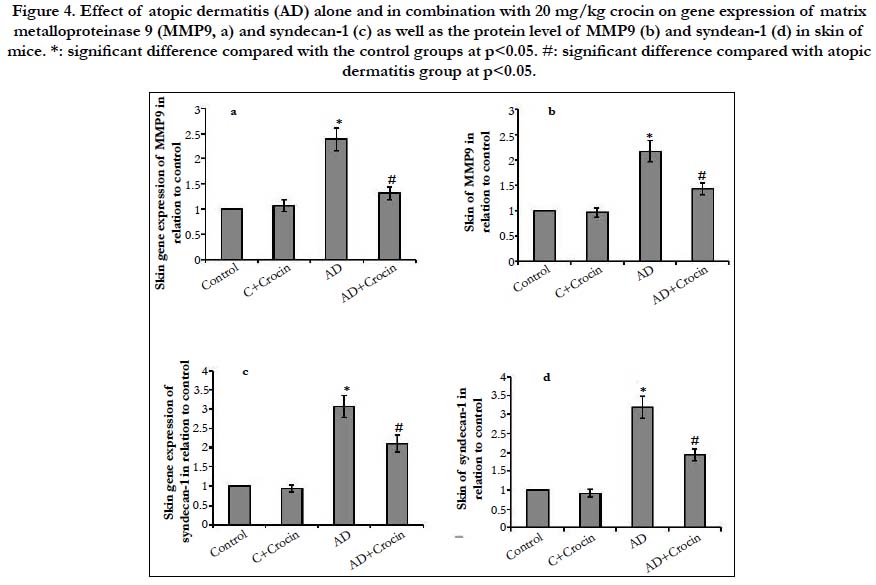
Skin of AD mice showed increased gene expression of the cell migration marker, AKT, MMP9 and syndecan-1 by 2.97-, 2.39- and 3.07-fold increase respectively in comparison with control mice (Figure 3 and 4). Moreover, AD resulted in elevated skin protein levels of AKT, MMP9 and syndecan-1 by 3.34-, 2.18- and 3.04-fold increase in comparison with control mice. Treatment of mice with Crocin decreased both gene and protein expression AKT, MMP9 and syndecan-1 in AD mice without affecting the control mice, however, their levels still significantly higher than control mice. The serine/threonine kinase AKT is a downstream effector that is activated by several stimuli, such as growth factor stimulation. AKT is a central component of the activated signaling pathway present in the endothelial cells that helps in epidermal terminal differentiation. Activation of AKT1 results in hyperkeratosis [18]. The posphrolyation of AKT activates its signaling pathway, therefore regulating the mast cell response and allergic disease [19]. The phosphorylation of AKT induces the transcription of many pro-inflammatory cytokines such as TNF-α and interleukins [20]. In addition, activation of AKT upregulates MMP9 enhancing cell invasion and migration [21]. In turn, MMP9 helps the proteolytic activation of syndecan-1 and releasing it in the surrounding media helping cell migration [22, 23]. AKT was previously reported to be involved in crocin activity on endothelium [24], against D-galactose aging model in the hippocampus of Wistar rats [25], angiogenesis in male rats [26] and on diabetic retinopathy in microglial Cells [27]. However, no previous study illustrated the effect of AKT/MMP9/syndecan-1 on atopic dermatitis.
Figure 3. Effect of atopic dermatitis (AD) alone and in combination with 20 mg/kg crocin on gene expression (a) and protein level of AKT (b) in skin of mice. *: significant difference compared with the control groups at p<0.05. #: significant difference compared with atopic dermatitis group at p<0.05.
Figure 4. Effect of atopic dermatitis (AD) alone and in combination with 20 mg/kg crocin on gene expression of matrix metalloproteinase 9 (MMP9, a) and syndecan-1 (c) as well as the protein level of MMP9 (b) and syndean-1 (d) in skin of mice. *: significant difference compared with the control groups at p<0.05. #: significant difference compared with atopic dermatitis group at p<0.05.
Conclusion
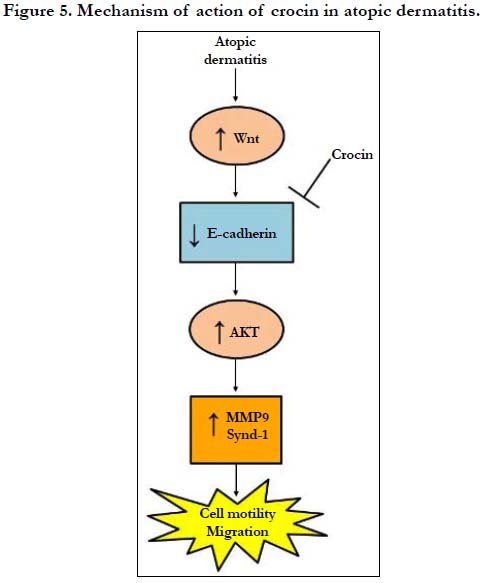
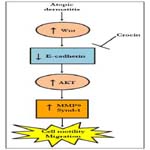
We illustrated that crocin could ameliorate DNCB-induced AD in mice. Crocin protection proceed via activation of E-cadherin leading to the inhibition of cell migration pathway (Figure 5).
References
- Ungar B, Garcet S, Gonzalez J, Dhingra N, Correa da Rosa J, Shemer A, et al. An Integrated Model of Atopic Dermatitis Biomarkers Highlights the Systemic Nature of the Disease. J Invest Dermatol. 2017; 137: 603-613. PMID: 27825969.
- Brunner PM, Silverberg JI, Guttman-Yassky E, Paller AS, Kabashima K, Amagai M, et al. (2017) Increasing Comorbidities Suggest that Atopic Dermatitis Is a Systemic Disorder. J Invest Dermatol. 2017 Jan; 137(1):18-25. PMID: 27771048.
- Sarkar R, Kanwar AJ. Atopic dermatitis. Indian Pediatr. 2002; 39: 922-930.
- Thestrup-Pedersen K. Clinical aspects of atopic dermatitis. Clin Exp Dermatol. 2000; 25: 535-543. PMID: 11122225.
- Nedoszytko B, Sokolowska-Wojdylo M, Ruckemann-Dziurdzinska K, Roszkiewicz J, Nowicki RJ. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014; 31: 84-91. PMID: 25097473.
- Silverberg JI, Simpson EL. Association between severe eczema in children and multiple comorbid conditions and increased healthcare utilization. Pediatr Allergy Immunol. 2013; 24: 476-486. PMID: 23773154.
- Yamazaki Y, Nakamura Y, Nunez G. Role of the microbiota in skin immunity and atopic dermatitis. Allergol Int. 2017; 66: 539-544. PMID: 28882556.
- Wollenberg A, Folster-Holst R, Saint Aroman M, Sampogna F, Vestergaard C. Effects of a protein-free oat plantlet extract on microinflammation and skin barrier function in atopic dermatitis patients. J Eur Acad Dermatol Venereol. 2018; 32 Suppl 1: 1-15. PMID: 29533490.
- Sung YY, Lee AY, Kim HK. The Gardenia jasminoides extract and its constituent, geniposide, elicit anti-allergic effects on atopic dermatitis by inhibiting histamine in vitro and in vivo. J Ethnopharmacol. 2014; 156: 33-40. PMID: 25153023.
- Park JH, Lee KY, Park B, Yoon J. Suppression of Th2 chemokines by crocin via blocking of ERK-MAPK/NF-kappaB/STAT1 signalling pathways in TNF-alpha/IFN-gamma-stimulated human epidermal keratinocytes. Exp Dermatol. 2015; 24: 634-636. PMID: 25868981.
- Alyoussef A. Arjunolic acid protects against DNCB-induced atopic dermatitis- like symptoms in mice by restoring a normal cytokine balance. Eur Cytokine Netw. 2015; 26: 38-45. PMID: 26553587.
- Alyoussef A. Suramin attenuated inflammation and reversed skin tissue damage in experimentally induced atopic dermatitis in mice. Inflamm Allergy Drug Targets. 2015; 13: 406-410. PMID: 26021324.
- Zhang YG, Wu S, Sun J. Vitamin D, Vitamin D Receptor, and Tissue Barriers. Tissue Barriers. 2013 Jan 1; 1(1). PMID: 24358453.
- Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33-driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. 2013; 210: 2939-2950. PMID: 24323357.
- Jiang A, Bloom O, Ono S, Cui W, Unternaehrer J, Jiang S, et al. Disruption of E-cadherin-mediated adhesion induces a functionally distinct pathway of dendritic cell maturation. Immunity. 2007; 27: 610-624. PMID: 17936032.
- Bakshi HA, Hakkim FL, Sam S, Javid F, Rashan L. Dietary Crocin Reverses Melanoma Metastasis. J Biomed Res. 2017. PMID: 29219852.
- Festuccia C, Mancini A, Gravina GL, Scarsella L, Llorens S, Alonso GL, et al. Antitumor effects of saffron-derived carotenoids in prostate cancer cell models. Biomed Res Int. 2014; 2014:135048. PMID: 24900952.
- Naeem AS, Tommasi C, Cole C, Brown SJ, Zhu Y, Benjamin Way, et al. A mechanistic target of rapamycin complex 1/2 (mTORC1)/V-Akt murine thymoma viral oncogene homolog 1 (AKT1)/cathepsin H axis controls filaggrin expression and processing in skin, a novel mechanism for skin barrier disruption in patients with atopic dermatitis. J Allergy Clin Immunol. 2017; 139: 1228-1241. PMID: 27913303.
- Kim MS, Radinger M, Gilfillan AM. The multiple roles of phosphoinositide 3-kinase in mast cell biology. Trends Immunol. 2008; 29: 493-501. PMID: 18775670.
- Kim WJ, Cha HS, Lee MH, Kim SY, Kim SH, Kim TJ. Effects of Cymbidium Root Ethanol Extract on Atopic Dermatitis. Evid Based Complement Alternat Med. 2016; 2016: 5362475. PMID: 26981139.
- Zeng Y, Yang Y. Piperine depresses the migration progression via downregulating the Akt/mTOR/MMP9 signaling pathway in DU145 cells. Mol Med Rep. 2018; 17: 6363-6370. PMID: 29488612.
- Al-Gayyar MM, Abbas A, Hamdan AM. Chemopreventive and hepatoprotective roles of adiponectin (SULF2 inhibitor) in hepatocelluar carcinoma. Biol Chem. 2016; 397: 257-267.
- Elewa MA, Al-Gayyar MM, Schaalan MF, Abd El Galil KH, Ebrahim MA, El-Shishtawy MM. Hepatoprotective and anti-tumor effects of targeting MMP-9 in hepatocellular carcinoma and its relation to vascular invasion markers. Clin Exp Metastasis. 2015; 32: 479-493. PMID: 25999065.
- Yang H, Li X, Liu Y, Li X, Li X, Wu M, et al. Crocin Improves the Endothelial Function Regulated by Kca3.1 Through ERK and Akt Signaling Pathways. Cell Physiol Biochem. 2018; 46: 765-780. PMID: 29621746.
- Heidari S, Mehri S, Hosseinzadeh H. Memory enhancement and protective effects of crocin against D-galactose aging model in the hippocampus of Wistar rats. Iran J Basic Med Sci. 2017; 20: 1250-1259. PMID: 29299203.
- Ghorbanzadeh V, Mohammadi M, Dariushnejad H, Abhari A, Chodari L, Mohaddes G. Cardioprotective Effect of Crocin Combined with Voluntary Exercise in Rat: Role of Mir-126 and Mir-210 in Heart Angiogenesis. Arq Bras Cardiol. 2017; 109: 54-62. PMID: 28678929.
- Yang X, Huo F, Liu B, Liu J, Chen T, Li J, et al. Crocin Inhibits Oxidative Stress and Pro-inflammatory Response of Microglial Cells Associated with Diabetic Retinopathy Through the Activation of PI3K/Akt Signaling Pathway. J Mol Neurosci. 2017; 61: 581-589. PMID: 28238066.