Comparative Effects Of Processing On The Cyanide Content Of Manihot Esculenta , Glycine Max And Zea Mays
Onyeike E.N, Nwaichi E.O*, Ibigomie C.E
Department of Biochemistry, University of Port Harcourt, Port Harcourt, P. M. B. 5323, Rivers State,Nigeria
*Corresponding Author
Nwaichi E.O,
Department of Biochemistry, University of Port Harcourt,
Port Harcourt, P. M. B. 5323,
Rivers State, Nigeria.
E-mail: nodullm@yahoo.com
Received: January 11, 2013; Accepted: January 25, 2013; Published: January 31, 2013
Citation: Nwaichi E.O, Onyeike E.N, Ibigomie C.E. (2013), Comparative
Effects of Processing on the Cyanide Content of Manihot Esculenta , Glycine
Max and Zea Mays. Int J Food Sci Nutr Diet. 2(1), 15-18. doi: dx.doi.org/10.19070/2326-3350-130004
Copyright: Nwaichi E.O© 2013. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The effects of varying processing treatments on the cyanide content of Manihot Esculenta,Zea Mays and Glycine Max were determined using picrate kit method and the following mean concentrations in ppm were obtained: 0.10, 0.00, 0.00, 0.00, 0.01, 0.00, 0.02,0.00, and 0.00 for Cassava, Garri, Fufu, Tapioka, Soybean, Vitamilk, Raw maize, Roasted maize, and Boiled maize respectively. There were statistically significant differences (P ≤ 0.05) among all raw samples analysed for the observed phytotoxin (Cyanide) levels. The results obtained from the processed and unprocessed food products generally showed a marked difference (P ≤ 0.05) in cyanide levels between raw and processed food products and this implies that food processing has a marked effect on the cyanide content of different food types indicating success in degradation of cyanide by heat. Heat treatments therefore reduced the cyanide content (approximate-ly 100%) in tested food crops thereby making them suitable and safer for consumption outside creating variety.
2.Introduction
3.Materials And Methods
3.1Sourcing and preparation of samples
4.Determination of the cyanide content in choice samples
5.Preparation of samples
6.Derivatization of glucose and maltose
7.Statistical Analysis
8.Results
9.Discussion
10.Conclusion
11.References
Keywords
Cyanide, Food processing, Heat treatment, Raw, Phytotoxin.
Introduction
Food crops are important sources of food for the human population and are used as food from which energy is derived to drive biochemical processes in the body anabolic and catabolic routes. In Nigeria, these food crops include cassava (Manihot escunlenta), maize (Zeamays) and soy ( Glycine Max), and individuals generates ATP (Adenosine triphosphate ) from the glucose content of these food crops. However, these food crops have some constituents like Cyanide (Okafor, 2004)that are toxic to mammals when harmful amount is consumed. Such levels could lead to serious side effects such as headaches, vomiting, chest pain, blood changes, thyroid gland enlargement, effects on the brain and heart, goiter and partial paralysis. These common food crops identified ultimately, are subjected to varying processing treatments before consumption by humans and needs evaluation vis – a – vis the potential processing effects on the cyanide content.
Some parts of cassava ( Manihot Esculenta) are edible such as the tuberous root and leaves. Most part of African countries, especially Nigeria uses this food crop as amajor source of food when it has been processed into garri, tapioca, flour (used in baking bread),andfufu. The soil in Africa is favourable for the cultivation of cassava since it is heat loving plantthat requires a minimium temperature of 80oF for its growth (Oke, 1978). The leaf of this plant is eaten as vegetable in parts of Africa and Asia due to its rich vitamin content (Oke, 1978). Some chemicals naturally present in food are cyanogenic glycosides (Okafor et al., 2001) from cassava, aflatoxin from food infected with the mould, aspergillus flavus, lysergic acid from grains contaminated by the mould clavicepspurpurea (Conn, 1973; Hartson, 1977; Lowenfels and Anderson, 1977; Obioda and Obonna, 1981).Cassava paste is packed into bags and tied tightly to remove all possible water content so that a dry form of the processed crop is obtained which is later transferred to a sieve to obtain fine powder particles that are fried at high temperature to produce garri. Cassava can also be used to produce ‘tapioca’ byboiling the clean peeled root in water to a temperature above 100oC.In the same vein, this applied heat destroys the cyanide present in the crop. The boiled cassava root is thensliced into small rectangular shaped por-tions and soaked in hot water for about 10hours. The rectangular shaped cassava rootobtained is packaged and sold as ‘tapioca’. MAIZE ( Zea Mays) also known as corn, is used widely as food in most part of Nigeria; it could be boiled or roasted to make it edible, the leafy stalk produces ears which contain seeds referred to as kernels. This food crop was carried from Europe to Africa in the 15th and 16thcenturies (Oke, 1978). It cangrow in different climatic conditions and has a sweet corn variety which is a sugar rich varietythatis mainly used as food to humans; field corn variety serves as feed to animals . Somechemicals such as cyanogenic glucosides, aflatoxin from food infested with the mould aspergillus flavins, lysergic acid from grains contaminated by the mouldclavicepspurpurea (Conn, 1973; Hart-son, 1977; Lowenfelsand Anderson, 1977; Obioda and Obonna, 1981 ) are naturally present in varying amount. This sweet corn is harvested early and eaten as a vegetable. For scientific reasons, maize is used in global context; in bulk trading however,corn is used as seen in the names of certain products made from maize such as popcorn, cornflakes and so on.
Maize can be ground into flour (Oke, 1978).Freshly harvested maize crops are assembled and packed into bags or othercontainers for transportation from the farm land to processing locations where the outer layers of the crop are removed to expose the grains attached to a stock.These grains are processed into different products depending on what is desired; it could be transferred into a pot and boiled to a temperature above 100oC for about 10 hours to obtain boiled corn. During the cookingprocess, salt is added to the crop to enhance its taste. The freshmaize could also be roasted on fire to give roasted corn, the covering layers of the maize plant are removed and the grains attached to stock exposed after which the maize is placed on a metal surface under which heat from char coal is applied to the maize through the openings on the metal surface. This applied heat changes the colour of the maize from white to light brown, and this is done for about 40minutes while turning to ensure even heating. SOY ( Glycine Max): Soy also known as Glycine Max or soy bean is a fabaceus leguminous food crop that is rich in proteindue to its rich essential amino acid content (Lawrence, 2010). It is used to produce Soy milk, infantformula, et cetera. it contains isoflavones that helps to pre -vent occurrence of osteoporosis, cardiovascular dis -eases as well as cancer (Oke, 1978). Soy contains a certain percentage ofcyanide sugars that are harmful to individuals when consumed at a toxic concen -tration. It is a good source of protein that contains small amount of saturated fats and does not con -tain cholesterol; this food crop is called soy bean by Americans and soyabean by the British, most Nigerians do call it soyabean where it is used for several milk products such as Vitamilk (Oke, 1978), powder and paste. Soy bean is an agricultural crop of tremendous economic importance; this crop and food items derived from it form dietary components of numerous people (Davut, 2011). Another product gotten from Soy isvegetable oil which is derived from processing and is widely used in industries.
Soy beans contains a reasonable amount of phytic acid, alphalinolenic acid,isoflavones,genistein and daidzen (Oke, 1978). Cyanide is a chemical that can exist as a gas or solid and that is poisonous when consumed orinhaled (CEC, 2006). It is a compound that is made up of cyano group –CN, that consist of triple bonded carbon atom to nitrogen. Most cyanides are highly toxic to mammals; it forms compound with other chemicals when attached to them such as hy-drogen cyanide , sodium cyanide and so on (Nartey, 1978 ). Some bacteria, algae and fungi do produce cyanide which also exist in food crops such as Manihot Esculenta (cassava), phaseolus Lunatus (Lima beans) commonly called Akidi by the Igbos in Nigeria, Glycine Max and maize ( Zea Mays ) (CEC, 2006).
Materials And Methods
The materials, reagents and chemicals used in this research work are of high quality and analar gradeand includecyanide buffer, cyanide reagent, cyanide indicator reagent, hydrochloric acid, sodium hydroxide, test tubes, 5ml glass caps, plastic spatula, plastic pipet (0.05ml), pH short range test paper (pH 9-14), plastic stirring rod, comparator, picrate acid and analysis kits
Samples used in this research work were purchased from different markets in Port Harcourt, Rivers State (mile 3 market, mile 1 market and Everyday super-market). These samples include freshly harvested cassava tuber, tapioca, fufu, garri, freshly harvested maize, roasted maize, boiled maize, soy bean, and vita milk (made from soy). The samples where cut into small portions and placed in a well labeled transparent container with cover.
Determination of the cyanide content in choice samples
Picrate kit method was used to determine the cyanide levels in certain food samples in a step by step analytical procedure that depends mainly on colour changes in the sample being analysed; each cyanide level has his own colour code from which the cyanide level in a particular sample can be determined through the following procedures – the portable balance was placed on its U-shaped plastic mount so that it swings freely, pestle and mortar were used to grind roasted or hard samples into powder. The process involves comparing a derived colour to the colours in a cyanogenic kit colour chart to determine the level of cyanide in each sample. The flour was added to an empty spatula until balance was gained. Developed colour was compared with the colour on the cyanogenic kit colour chart which indicated the presence or abscence of cyanide and the concentration if present.
Preparation of samples
Exactly 0.1 g of honey was weighed and dissolved with 100 ml of pure water.
Derivatization of glucose and maltose
To 5 ml of the water solution of honey, 400 μl of 1.4 M sodium cyanoborohydrate solution in water, 400 μl of acetic acid, and 2 mL of 0.6 M ethyl ester aminobenzoic acid (methanol) were added, and the mixture was heated at 800C for 10 minutes. After it had been cooled to room temperature, 2 ml of distilled water was added. The water phase was washed with 4 ml of chloroform to remove ethyl ester of aminobenzoic acid from the water phase, and the water phase was applied to HPLC.
Statistical Analysis
Means and standard errors of triplicatedeterminations were calculated and tabulated. Analysis of Variance, ANOVA was done using EXCEL WINDOWS 2003.
Results
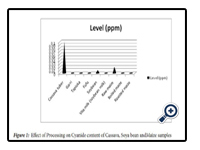
The levels (ppm) of cyanide in Garri, Cassava tuber, Maize, boiled maize, roasted maize, tapioca, fufu, and vitamilk are as shown in table 3.1. Detectable ranges of 0.01ppm to 0.10ppm were obtained for Cassava tuber, soya bean and raw maize while cassava tuber ranked highest among all measured samples.
Discussion
Availability of cyanide in choice food items and ef-fects of processing was evaluated by picrate kit method which involves comparing derived colours to the colours on a cyanide comparator colour chart. Nigerian staple food crops (Cassava and Maize) and Soya bean gave phytotoxin (cyanide) values that ex-ceeded WHO limit (0.00 ppm) for cyanide in food in food materials. Food crops are important sources of food nutrients (Lawrence, 2010), energy, protection and repair to the human population. The processing of food crops into food products has a significant ef-fect on the properties of the food in terms of taste, aroma and colour, as well as changes in the chemical compounds present in such a food such as cyanide (Bo urdoux et al., 1980), which is a chemical that can exist as a gas or solid, and that is poisonous to the body when consumed or inhaled. The cyanide val -ues obtained from the different samples in this work showed 100% degeneration by heat treatment for the different processed food samples (Fig 3.1).Processing, therefore reduced the cyanide content in food crops thereby making them suitable and safer for consumption outside creating variety. Significant elavated levels of 0.1ppm cyanide content was observed for raw cassava tuber. Two cyanogenicglycosides, Linamarin and Lotaustralin occur generally in manihotspecies. Linamarin accounts for 93-97% of the total cyanogenic glycoside content of cassava (Nartey, 1978). When an individual is exposed to high levels of cyanide, his brain and heart could be affected, chest pain, vomiting, blood discharges, thyroid gland enlargement, liver damage and headaches are all effects of exposure to cyanide (Ekpechi, 1973; Hartley et al., 1963). There were statistically significant differences (P ≤ 0.05) among all raw samples analysed (Table1). The results obtained from the processed and raw samples implies that varying food processing has a marked effect on the cyanide content of different tested food types; thus indicating success in degradation of cyanide by heat.
Values are mean ± SE of triplicate determinations. Means in the same colum with the same alphabet are not significantly different (P ≤ 0.05).
Conclusion
Food Processing has a marked effect on the cyanide content of food crops as was evidentin this work. Presence of cyanide was observed in raw food samples while there was no trace of cyanide in processed samples, which indicates the elimination of cyanide from the raw samples by various processing methods. Food processing should be adequately done to reduce the possibility of cyanide intake by individuals so as to prevent inherent toxicity and to drive good public health.
References
- Anosike, E. O. and Alaso S. J. (1982). Kidney Rhoda -nese from the Guinea Pig ( Lepus Caniculus ) and Albino Rat ( Mus musculus ). 27 (1): 33 – 39. www.ncbi.nlm.nih.gov/pub-med/6950896. Retrieved on 23rd July 2012.
- Anosike, E. O., Uwakwe, A. A., Monanu, M. O. and Ekeke, G. I. (1991). Studies on Human Erythrocyte Glu-tathione – S -Transferase from HbAA, HbAS and HbSS. Biomedi-cal Biochemistry Acta. 50 (9): 1051 - 1056.
- Anosike, E. O., Eminedoki, D.G., and Monanu, M.O. (1994). Thiocyanate levels of mainly dietary origin in Serum and Urine from a human population sample in Port Har-court. Plant Foods. Human Nutrition. 46: 277-285.
- Anosike, E. O. and Ugochukwu U.N. (1981). Characteri -zation of Rhodanese from Cassava leaves and tubers. Jour-nal of Experimental Botany. 32 : 1021-1027.
- Auriga, M. and Koj, A. (1975). Protective Effects of Rhodanese on the Respiration of Isolated Mitochondria In-toxicated with Cyanide. Bulletin of Academy of Political Science (Biology). 23: 305-310.
- Barrett, M. P. D., Alexander, J.C. and Hill, D.C. (1978). Utilization of Samples from Radioactive Methionine or Sul-fate in the Detoxification of Cyanide by Rats. Nutritional Me-tabolism. 22: 51-57.
- Bolhius, G. G. (1954). The Toxicity of Cassava.Agricul -tural Science. 2: 176-185.
- Bourdoux, P., Mafuta, M., Harson, A. and Ermans, A. M. (1980). Cassava Toxicity, the role of linamarin. In: Role of Cassava in the Etiology of EndemicGoitre and Cretinism. In: Ermans, A. M., Ambulamoko, N. M., Delange, F. and Ahlu -walia. Revised Ed.15 - 27.
- Boyd, E.M., Boulanger, M. A. and De Castro, E.S. (1969). Phenacetin Toxicity and Dietary Protein. Pharma-cology.15-19.
- CEC. (2006). Commissionof the European Communi -ties.Setting maximum levels for certain contaminants in foodstuffs. 1881.
- Conn, E.E. (1969). Cyanogenic glycosides. Agricultural Food Chemistry. 17: 519-520.
- Conn, E.E. (1973). Cyanogenic glycosides, their occur -rence, biosynthesis and function. In; Chronic cassava toxicity. Nestel, D. and Macintyre, R. IDRC, Ottawa, ON, CA Publishers. 55-63.
- Cooke, R.D. and Maduagwu, E.N.(1978). Effects of simple processing on the cyanide content of cassava chips. Technology. 13: 299-306.
- Delange, F. and Ermers, A. M. (1971). Role of a dietary goitrogen in the etiology of endemic goiter on Idiwi Is-land. Clinical Nutrition. 24: 1354-1360.
- Delange, F., Van, D., Velden, M. and Ermans, A.M. (1973). Evidence of antithyroid action of cassava in man and in animals. In: Chronic cassava toxicity, Nestel, B., Macin-tyre, R. IDRC, Ottawa, ON, CA Publishers. 147- 151.
- Dixon R. L., Shultice, R.W. and Webb, E.C. (1958). En -zymes. Academic press, New York. 12 - 23.
- Dixon R.L., Shultice, R.W. and Fouts, J.R. (1960). Factors affecting drug metabolism by liver microsomes in starvation. Production Society for Experimental and Biological Medicine. 103: 333-335.
- Eyjolfson, R. (1973). Recent advances in the chemistry of cyanogenic glycosides. Chemical organization Naturalist. 28: 74-108.
- Ekpechi, O. L. (1973). Endemic goiter and high cassava diets in Eastern Nigeria. In: Chronic cassava toxicity. IDRC, Ottawa, ON, CA publishers.139-145.
- Frendo, J. and Dudek, M. (1978). 3-Mercaptopyruvate sulphurtransferase and rhodanese activities in developing chick embryo.Folia Biologica. 26 (3): 209-215.
- Feldstein, M. And Klendshoj,N.C. (1954). Industrial hy -giene and toxicology.Second revised edition. Intersci-ence Publishers, New York. 1991-1999.
- Fassette, D.W. and Naish, D.D. (1963). Industrial Hygiene and toxicology. Inter science publishers, New York. 1991-1999.
- Gibb, M.C., Carbery, J.T., Carter, R. G. and Catalinac, S. (1974). Hydrocyanic acid poisoning of cattle associated with su -dax grass. Veterinary Journal. 22 (7): 127.
- Halstrom, F. and Moller, K. D. (1945). Content of cya -nide in human organs from cases of poisoning with cyanide taken by mouth, with contribution to toxicology of cya-nides. Acta Pharmacology. 1: 18 -28 .
- Hartley, R.D., Nesbitt, B.F. and O’Kelly, J. (1963). Toxic metabolites of aspergillusflavus. Nature. 198: 1056 – 1058.
- Hartson, E.A. (1977). Food and drug interaction. Jour -nal of animal diet association. 70 (1): 16 - 19.
- Lang, K. (1933). Die rhodanbilding in tierkorpor. Bio -chemistry. 259: 213 – 256.
- Lowenfels, A. and Anderson, M.E. (1977). Diet and can -cer. Cancer. 39: 1809 – 1814.
- Maduagwu, E.N. (1979). Cyanide content of garri. Toxi-cology. 3 (1): 21 -24.
- Meister, A. (1953).Preparation and enzymic reactions of the ketoanalozines of asparagine and glutamine. Federal production.12 : 245.
- Montgomery, R.D. (1964). Observations on the cya-nide content and toxicology of tropical pulses. West Indi-ans Medicine Journal. 13: 1 – 11.
- Montgomery R.D. (1965).The medical significance of cyanogen in plant food stuff.Animal journal on clinical nutrition.17: 103 -113.
- Montgomery, R. D. (1969). Cyanogens. In: Toxic con-stituents of plants food stuff. Lienor Academic press New York. 143 -157.
- Muller, Z., Chou, K.C. and Nah, K. C.(1975). Cassava as a total substitute for cereals in livestock and poultry ra -tions. Proceedings of the 1974 Tropical Products Institute Conference. 1 – 5 April. 85 - 95.
- Nartey, F. and Moller, B. L. (1976). Amino acid profiles in cassava seed and seedlings. Tropical root crops. 23-29.
- Nash, T. (1963). The colorimetric estimation of formal -dehyde by means of the hantzch reaction. Biochemistry jour-nal. 55: 416 - 421.
- Obioda, O. and Obonna, E. E. (1981). Aflatoxin inhibi-tion of reversed electron transfer in rat liver mitochon -dria. 26: 1 -7.
- Okafor, P. N. (2004). Assessment of cyanide overload in cassava consumingpopulations of Nigeria and the cyanide con-tent of some cassava based foods. African Journal of Biotech-nology. 3 (7): 358-361.
- Okafor, P. N., Okoronkwo, C.O., Alaneme, F. O., and Maduagwu, E.N. (2001).
- Cyanide contamination of natural water sources dur -ing large scalecassava processing. African Journal of Biomedical Research. 4: 25-27.
- Oke, O. L. (1968). Cassava as food in Nigeria. World re-view on nutrition and diet. 1: 277 – 250 .
- Oke,O.L. (1973). The mode of cyanide detoxification. In : Chronic cassava toxicity. Nestel, B. and Maclntyre, R. IDRC, Ottawa, ON, CA Publishers. 97 -104.
- Osuntokun, B.O. (1973). Ataxic neuropathy associated with high cassava diets in West Africa. In: Chronic cassava toxic-ity. Nestel, B. and Maclntyre, R. IDRC, Ottawa, ON, CA Publishers.127 -138.
- Reinwein, D. (1961). Die Verteilung der thiosul -fatschwefeltransferase. Physiological chemistry. 326: 94 – 101.
- Sorbo, B. H. (1975). Thiosulphatetransferase and mer -captopyruvatesulphurtransferase.In; Metabolic pathways. Aca-demic press, New York.11: 433 – 456.
- Tewe, O.O. and Manner, J.H. (1981). Long term effects of dietary inorganic cyanide in the life performance and metab-olism of rats. Pharmacology. 58: 1-7.
- Wokes, F., and Baster, N. (1975). Effects of light on vita-min B12.Biochemistry journal. 53: 29 – 34.
- Wheeler, J. L., Hedges, D. and Till, J. (1975). A possible effect of cyanogenic glycoside in sorghum on animal require -ments for sulphur. Journal on agricultural science. Cambridge. 84: 377 -379.