Protective Effect on Lipids Profile of Lion’s Foot (Alchemilla Vulgaris) Leaves against Ccl4 Toxicity and its Fortified to Guava and Mango Pulp
El-Hadidy EM1, Refat OG2, Halaby MS2, Elmetwaly EM3, Aya A.A. Omar3*
1 Food Technology Research Institute, Agriculture Research Center, Giza, Egypt.
2 Faculty of Home Economics, Nutrition and Food Sciences Department, Helwan University, Cairo, Egypt.
3 Faculty of Girls, Nutrition and Food Sciences, Home Economics Department, Ain Shams University, Cairo, Egypt.
*Corresponding Author
Aya A.A. Omar,
Faculty of Girls, Nutrition and Food Sciences,
Home Economics Department, Ain Shams University, Cairo, Egypt.
Tel: +20 01003299652
E-mail: aya.gad@women.asu.edu.eg
Received: December 26, 2018; Accepted: January 11, 2019; Published: January 12, 2019
Citation: El-Hadidy EM, Refat OG, Halaby MS, Elmetwaly EM, Aya A.A. Omar. Protective Effect on Lipids Profile of Lion’s Foot (Alchemilla Vulgaris) Leaves against Ccl4 Toxicity and its Fortified to Guava and Mango Pulp. Int J Food Sci Nutr Diet. 2019;8(1):394-400. doi: dx.doi.org/10.19070/2326-3350-1900070
Copyright: Aya A.A. Omar© 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Lion’s foot (Alchemilla vulgaris L.) which is widely known as lady’s mantle, bear’s foot or lion’s foot is traditionally used due to their tannin contents for the treatment of inflammations, diarrhea, wound healing and inhibit cardiovascular diseases and cystic fibrosis. The present work aims to high light the reverse effect of dried lion’s foot (Alchemilla vulgaris) leaves and its water and ethanol extracts at various ratios against the toxicity of CCl4 on serum lipids profile that may occur on albino rats. Also, preparation of guava and mango pulp that fortified with lion’s foot powder and its water and ethanol extracts as an available product rich in bioactive component to help hyperlipidemic patients. Fifty-six male albino rats were used in this experiment. These rats were divided into eight main groups (seven rats of each) and were fed on diets for 45 days as follows: Group 1: Negative control group was fed on basal diet. Forty nine rats were fed on basal diet and treated with CCl4 subcutaneous injection to induce toxicity, then divided into 7 groups form group 2 to group 8. Group 2: Positive control group will be fed on basal diet till final experiment. Groups 3:8 as the same of the second group with (50 and 100 ppm ethanol extract, 50 and 100 ppm water extract, 1% and 2% dry leaves powder respectively) daily orally. Moreover, guava and mango pulps were classified to 7 groups as follows: control sample, and the samples (2 to 7) were fortified with lion’s foot as follows ethanol extract 50ppm and 100ppm , water extract 50ppm and 100ppm, 1% and 2% dried leaves for sensory evaluation. All these samples were organoleptically evaluated for general appearance, color, flavor, taste, consistency and overall acceptability.
Results: The results revealed that treatment with lion’s foot leaves and its ethanol and water extracts caused significant decreased levels of serum lipid profile of total cholesterol, triglycerides, total lipids, LDL-C and VLDL-C induced by CCl4 and enhancement HDL-C in all groups. The best result of lipid profile was in groups treatment with the high level 2% dried lion foot followed 1 % dried lion’s foot and 100ppm ethanol extract. These results due to the antioxidants content in Lion’s foot as polyphenols, flavonoids, tannins and saponins (359.65, 183.10, 150.64 and 296.32, respectively). The best sample of fortified guava pulp was 1% dried leaves. While, the best result of mango pulp was in the samples fortified with 50ppm ethanol extract, water extracts (50 and 100 ppm) and 1% dried leaves.
Conclusion: This study showed that the fortification of Alchemilla vulgaris L. can be proposed to protect the toxicity induced by CCl4 in rats, also to help inhibit the improvement cardiovascular diseases and cystic fibrosis.
2.Abbreviations
3.Introduction
4.Materials and Methods
4.1 Materials
4.2 Methods
4.2.1 Preparation of extract
4.2.2 Determination of Antioxidants content
4.2.3 Diet composition and animal groups
4.2.4 Experimental design
4.2.5 Serum sampling
4.2.6 Biochemical analyses of serum
4.2.7 Guava and mango pulp preparation
4.2.8 The sensory parameters
4.2.9 Statistical analysis
4.Results
5.Discussion
6.References
Keywords
Lion’s Foot (Alchemilla Vulgaris); Dried Leaves; Water and Ethanol Extract; CCl4 Cytotoxicity; Lipids Profile; Hyperlipidemic; Mango and Guava Pulp.
Abbreviations
GAE: Gallic Acid Equivalents; QE: Quercetin Equivalents; ANOVA: Analysis of One Way Variance; MDA: Malondialdehyde.
Introduction
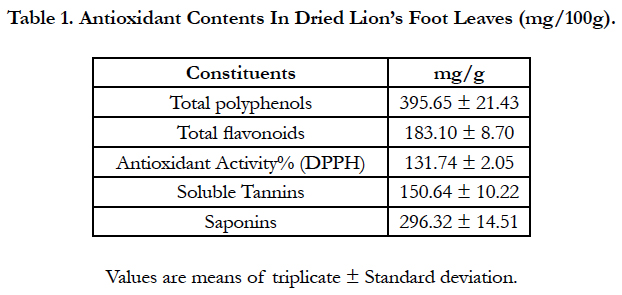
Lion’s foot (Alchemilla vulgaris L.)which is widely known as lady’s mantle, bear’s foot or lion’s foot is traditionally used due to their tannin contents for the treatment of inflammation of the upper digestive tract, diarrhea internally and as wound healing and astringent externally [1] and [2]. Another use of Alchemilla Species is for the adaptation to the hormonal levels of the body in case of menopause [3]. Reference [4] revealed that antioxidants content in dried lion’s foot leaves (mg/100g) was total polyphenols 395.65 ± 21.43, total flavonoids 183.10 ± 8.70, antioxidant activity% (DPPH) 131.74 ± 2.05, soluble tannins 150.64 ± 10.22 and Saponins 296.32 ± 14.51. Also, benzoic acid, ellagic acid, catechol, catechin, ellagic acid, salicylic acid and vanillic acid recorded the highest contents which known with their antioxidant and antiinflammatory properties [5]. Lady’s Mantle has been described as an important herb for the cleansing and healing of topical and internal wounds (ulcers, festering wounds and shot wounds), to diffuse internal blood clots, for ulcers and inflammation in the mouth and throat. The pulped leaves, he says, can be applied directly or via a linen cloth soaked in the juice of the leaves [6] and [7]. Reference [8] reported that carbon tetrachloride (CCl4) is assumed to initiate the biochemical processes leading to oxidative stress, which is the direct cause of many pathological changes in liver, kidney, testes, lungs, nervous system and blood tissues by producing free radicals. These free radicals frequently damage different cell organelles through lipid peroxidation.
Antioxidants and anti-inflammatory agents play a critical role against CCl4 intoxication by scavenging active oxygen and free radicals and neutralizing lipid peroxides [9] and [10]. Catechin reduced the total cholesterol, and LDL cholesterol [11]. Reference [12] revealed that the phytochemicals ellagic acid showed hepatoprotective activities in CCl4 - induced hepatotoxicity in mice. The protective action was improved further by doubling the dose of the phytochemicals. Apart from the anti-lipid peroxidative and antioxidant actions, these active phytochemicals might have played a role in restoring the cytochrome P450 enzyme system or promoted the liver regenerative activity.
The aim of the present work was to high light the reverse effect of dried lion foot and its water and ethanol extracts at various ratios against the toxicity of CCl4 on serum lipids profile that may occur on albino rat. Also, to determine sensory evaluation of guava and mango pulp that fortified with lion’s footwater, ethanol extracts and dry powder.
Lion’s foot (Alchemilla vulgaris) leaves was obtained from Horticulture Research Institute, Agriculture Research Center Giza, Egypt. While, Guava (Psidium guajava L.) and Mango (Mangiferai ndica L.) fruits were obtained from private farm. Carbon tetrachloride (CCl4), casein, vitamins, minerals, cellulose and choline chloride will be purchased from El-Gomhoreya Company, Cairo Egypt. Corn oil and starch were obtained from the local market. Fifty six male albino rats (Sprague Dawley strain) weighing an average (200 ± 10g) were obtained from animal house in Food Technology Research Institute, Agriculture Research center, Giza - Egypt. Serum cholesterol, triglycerides, HDL, LDL, and VLDL kits were purchased from Biodiagnostic Company in Egypt. Guava and mango were obtained from local markets.
Lion’s foot were washed in water several times to remove any adhering flesh, dried in oven under vacuum, then ground well. Ground lion's foot was dipping in ethanol 80% (1:100 w/v) in dark bottle for 48h at ambient temperature (25°C) to obtain extracts, and then mixtures were filtered by filter paper (Whatman1). Water solution was evaporated at 55°C in rotary evaporator, while ethanolic extract was evaporated 40°C till completely evaporation.
Total phenolic content was determined by the Folin-Ciocalteu method of [13]. The extracted samples were mixed with Folin-Ciocalteu reagent for 6 min, then 7% sodium carbonate (NaCO3) was added. The absorbance was measured at 760 nm after 90 min of incubation at room temperature. Results were expressed as mg gallic acid equivalents (GAE) per 100 g sample. While, total flavonoids were determined using the method of [14]. Briefly, 0.5 ml of sample, 0.5 ml of 2% AlCl3 and methanol solution was added. After 1 h at room temperature, the absorbance was measured at 420 nm. Total flavonoids content was calculated as quercetin equivalent from a calibration curve and expressed as quercetin equivalents (QE) per 100g. Total tannins content (mg/100g) was evaluated according to the method of [15]. Saponins were determined by the method of [16]. The antioxidant activity was determined using the 1,1diphenylpicrylhydrazyl (DPPH) assay described by [17].
Diet composition: Basal diet was prepared according to [18]. The vitamin and mineral mixture had the prepared according to [19].
In animal house in Food Technology Research Institute, Agriculture Research Center; albino rats were adapted for one week prior to commencement of the experiment, housed in well aerated cages under hygienic condition and water was introduced ad-libitum. After this week, rats were divided into 8 main groups (seven rats of each) and fed on diets for four weeks as follows: Group 1: Negative control group fed on basal diet. Forty nine rats fed on basal diet and treated with CCl4, in paraffin oil (50% v/v 2 ml/kg) twice weeks subcutaneous injection to induce chronic damage in the liver [20], 7 groups numbered from 2 to group 8 were classified as follows: Group 2: Positive control group had fed on basal diet till final experiment. Group 3: treated as group 2 with 50 ppm ethanol extract daily orally. Group 4: treated as group 2 with 100 ppm ethanol extract daily orally. Group 5: treated as group 2 with 50 ppm water extract daily orally. Group 6: treated as group 2 with 100 ppm water extract daily orally. Group 7: treated as group 2 with 1% leaves powder in diet. Group 8: treated as group 2 with 2% leaves powder in diet.
At the end of the experiment period, the rats were fasted overnight then the rats were anaesthetized, sacrificed and blood samples were collected from the aorta. The blood samples were centrifuged for 15 minutes at 3000 rpm to separate the serum. The serum was carefully separated into dry clean Wassermann tubes by using a Pasteur pipette and kept frozen till analysis at -20°C.
Serum lipid profiles: Serum samples were used for the determination of total cholesterol, triglycerides, Total lipids,and HDL-C, according to the methods described by [21]. Serum LDL-C, and VLDL-C were calculated according to the following equation:
1. VLDL-C Concentration (mg/dL) = TG (mg/dl)/5
2. LDL-C = Total Cholesterol – [(VLDL-C) + (HDL-C)]
Guava and mango were washed, then mango fruits were peeled. The guava pulp was extracted by using pulper machine and strained through 1 mm stainless steel sieves to remove seeds. Guava or mango pulps were blended in the mixer without any additives such as (water and sugar). Guava and mango pulps were placed in transparent glass bottles and classified to 7 groups as follows: control sample, and the samples (2 to 7) were fortified with lion’s foot as follows: 50ppm and 100ppm ethanol extract, 50ppm and 100ppm water extract, and 1% and 2% dried leaves.
The sensory parameters of appearance, colour, flavour, taste, consistency and acceptability were evaluated by 10 trained panelist based on 10 point Hedonic rating scale with maximum score considered as the best [22].
Results were analyzed using SPSS 19.0 Program (2000). Means and standard deviations were determined using descriptive statistics. Comparison between means of samples was determined using analysis of one way variance (ANOVA) and multiple range tests. Statistical significance was defined at P ≤ 0.05.
Results
Tables and Figures given below.
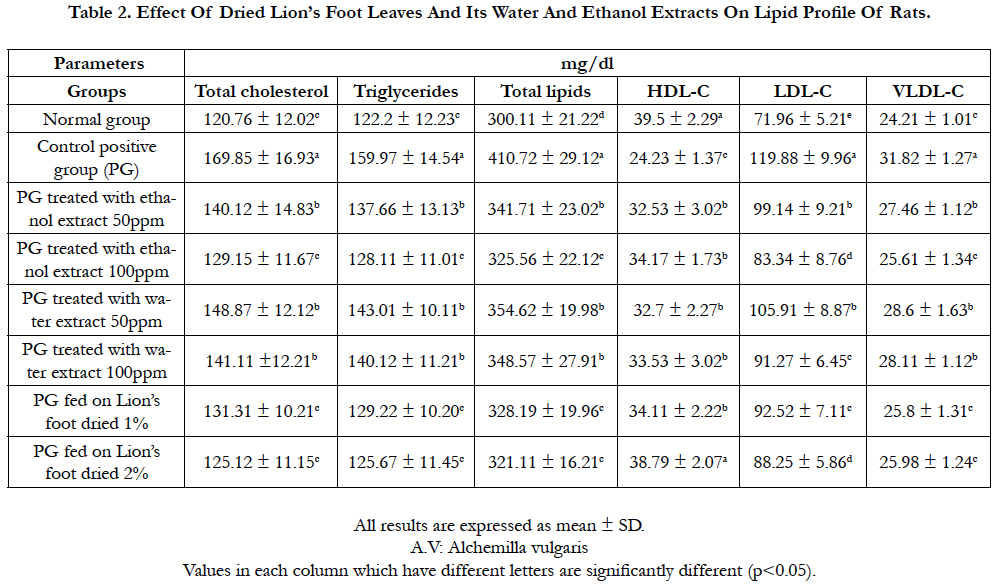
Table 2. Effect Of Dried Lion’s Foot Leaves And Its Water And Ethanol Extracts On Lipid Profile Of Rats.
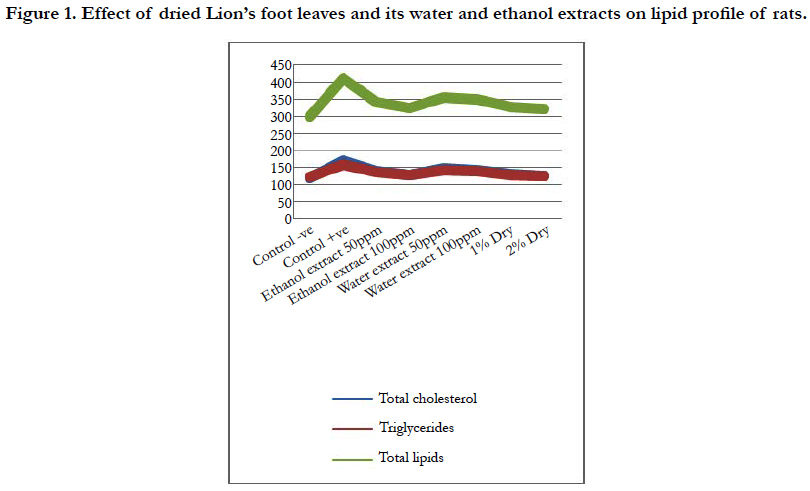
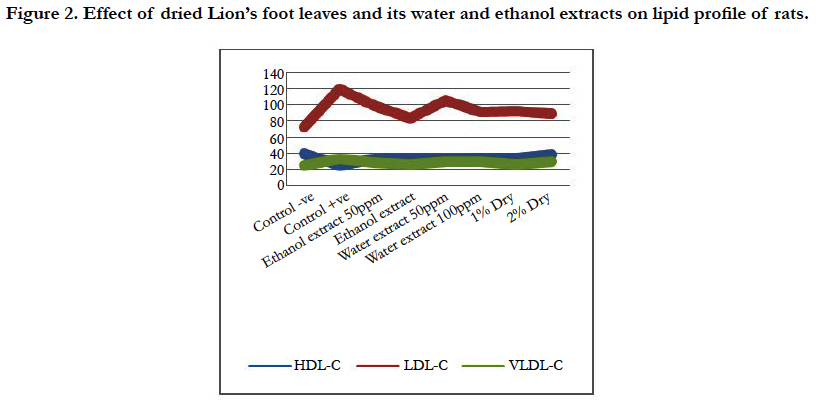
Figure 1. Effect of dried Lion’s foot leaves and its water and ethanol extracts on lipid profile of rats.
Figure 2. Effect of dried Lion’s foot leaves and its water and ethanol extracts on lipid profile of rats.
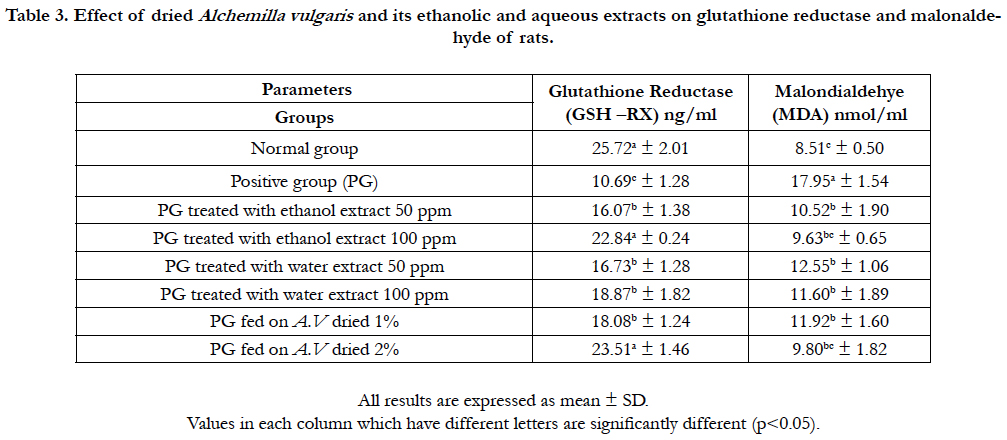
Table 3. Effect of dried Alchemilla vulgaris and its ethanolic and aqueous extracts on glutathione reductase and malonaldehyde of rats.
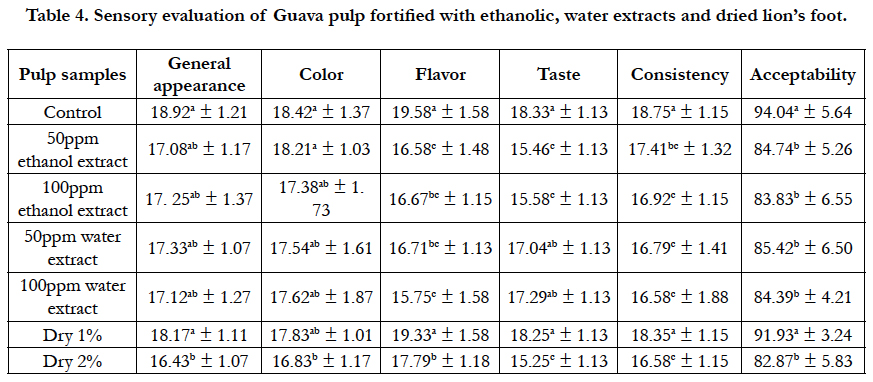
Table 4. Sensory evaluation of Guava pulp fortified with ethanolic, water extracts and dried lion’s foot.
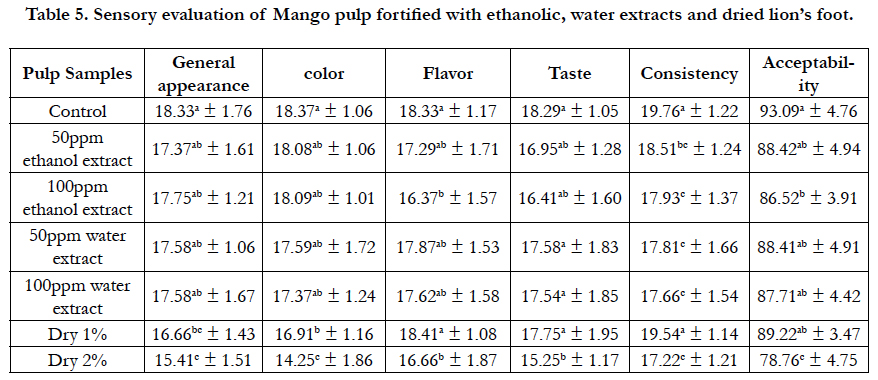
Table 5. Sensory evaluation of Mango pulp fortified with ethanolic, water extracts and dried lion’s foot.
Discussion
Total polyphenols, total flavonoids, soluble tannins, saponins and antioxidant activity of dried lion’s foot are recorded in Table 1. The data indicated that dried lion’s foot leaves had rich in total polyphenolic and flavonoids content (395.65 and 183.10 mg/100g, respectively). Also, it’s observed the highest content of soluble tannins and saponins (150.64 and 296.32 mg/100g) in dried leaves. These results were reflected to the antioxidant activity, its noticed that the antioxidant activity of dried lion’s foot leaves was high (131.74%). European Pharmacopoeia described the aerial parts of lion’s foot and its chemical content as containing a minimum of 6% tannin, chlorogenic acid and caffeic acid [23]. The effects of lion foot leaves and its water and ethanol extracts on serum lipid profile of rats toxic with CCl4 are shown in Table 2.
Rats fed on diets containing 2% and 1% dried leaves were highly significant decrease in serum total cholesterol (125.12 and 131.31mg/dL, respectively), followed by rats fed on ethanol extract 100 and 50 ppm orally (129.15 and 140.12 mg/dL, respectively), then rats fed on water extract 100 and 50 ppm orally (141.11 and 148.87mg/dL, respectively) compared to positive control group (169.85 mg/dL). Data indicated to rats fed on 2% dried lion’s foot was significant decrease in serum total cholesterol, also rats fed on 100ppm ethanol and water extract followed by rats fed on 50 ppm extractions orally (Table 2).
In Parallel, rats fed on diets containing 2% and 1% dried lion’s foot were highly significant decrease in serum triglycerides (126.67 and 129.22 mg/dL, respectively) and serum total lipids (321.11 and 328.19 mg/dL, respectively) then rats fed on 100 ppm ethanol and water extract orally, either serum triglycerides or total lipids (Table 2, Figure 1 ).
The level of serum HDL in rats after treated to 2% dried lion’s foot (38.79 mg/dL) was nearly to rats fed on normal control group (39.50 mg/dL), also the same rats group fed on 2% dried lion’s foot was highly significant increase in serum HDL compared to rats fed on positive control diet (24.23 mg/dL). Also, Another rats groups either fed on ethanol or water extracts orally were significant increase in serum HDL compared to rats fed on positive control diet (Table 2, Figure 2 ).
In contrast, rats fed on diets containing 2% and 1% dried lion’s foot were highly significant decrease in LDL and HDL, followed by rats fed on 100 and 50 ppm ethanol extract orally, then rats fed of 100 and 50 ppm water extract orally compared to rats fed on positive control diet (Table 2, Figure 2).
Conclusively, serum total cholesterol, triglycerides, total lipids, LDL and VLDL were significant decrease in rats fed on diet containing dried lion’s foot and rats fed on either ethanol or water extracts, orally (Figure 2), while HDL was significant increase. Also, toxicity with CCl4 caused significant rise in cholesterol, triglycerides, total lipids, LDL-C and VLDL-C while, there was significant decrease in HDL-C.
These results are may due to the content of phyotchemicals as antioxidant and its activity (DPPH) in lion’s foot which have a significant effect on lowering all lipids parameters except increase in HDL level. These data were in agreement of [4] and [11]. It could be noticed from the presented data in Table (3) that positive control (induced with CCl4) showed a significant decrease of glutathione reductase activity (10.69ng/ml) compared to rats fed on basal diet (25.72ng/ml). It’s clear rats fed on diet containing 2% lion’s foot and 100ppm ethanol extract were highly significant increase in glutathione reductase (23.51 and 22.84 ng/ml, respectively) while, there was significant increase of other treated roups.
On the other hand, data showed marked increase in the serum Malondialdehyde (MDA) level of rats fed on the positive control group (17.95 nmol/ml) compared with normal group (8.5 nmol/ ml). Rats in groups treated with dried lion’s foot and its ethanol and water extracts recorded a significant decrease in the serum MDA level. The best result was observed in the rats groups treated with 100ppm extract and 2% powder (9.80 and 9.63 nmol/ml, respectively). These data may due to polyphenols and flavonoids content in lion’s foot which effect working as a scavenging free radical to prevent liver cell damage [24]. Sensory evaluation scores of fortified Guava pulp with different levels of Alchemilla Vulgaris leaves powder (1% and 2%) and its extracts of water and ethanol (50ppm and 100ppm) are shown in Table (4). It was found that, there was no significant difference (P0.05) of general appearance and color between control sample and all fortified samples except a sample fortified with 2% dry leaves powder.
On the other hand, table (4) showed a significant statistical difference between control sample and all fortified guava pulp samples in flavor evaluation except a sample fortified with 1% dry leaves powder which recorded (19.33 ± 1.58) compared with the control pulp sample (19.58 ± 1.58).
The best results of taste evaluation were in pulp samples fortified with 50ppm water extract, 100ppm water extract, and 1% dry leaves powder. The values were (17.04 ± 1.13, 17.29 ± 1.13, and 18.25 ± 1.30, respectively) compared with control sample (18.33 ± 1.3) which recorded no significant difference between them. While, there was significant statistical difference between control sample and the samples fortified with 50ppm ethanol extract, 100ppm ethanol extract, and 2% dry leaves powder of lion’s foot indicated that acceptable sweet taste which can be taken on its own or in a herbal combination [7].
Concerning the consistency of guava pulp, there were significant differences between all fortified groups and control sample except 1% dry lion foot leaves powder that showed no significant difference compared with control sample.
Fortification with dried lion’s foot leaves and its extracts of water and ethanol on acceptability are shown also in Table (4). It was found that there was no significant statistical difference in sample pulp fortified with 1% dry leaves powder (91.83 ± 3.24) compared to control sample (94.00 ± 5.64) that recorded the best sample of fortified pulp followed by the other samples where there were no statistical differences between there samples., the values were (84.74 ± 5.26, 83.83 ± 6.55, 85.42 ± 6.50, and 82.87 ± 5.83, respectively). Sensory evaluation scores of fortified mango pulp with different levels of lion foot leaves powder (1% and 2%) and its extracts of water and ethanol (50ppm and 100ppm) are shown in Table (5). It was found that,there was no significant difference in general appearance and color between control sample and (50ppm and 100ppm) water extract samples while, there was significant statistical difference between control sample and the samples fortifies with 1% dry and 2% dry lion foot. Concerning flavor of mango pulp, there was no significant difference between control sample mango pulp and the samples fortified with 50ppm ethanol extract, 50ppm water extract, 100ppm water extract and 1% dry. While, there was significant difference between control sample and the samples fortified with 100ppm ethanol extract and 2% dry.
The best results of taste were in pulp samples fortified with (50ppm and 100ppm) water extract, 1% dry followed (50ppm and 100ppm) ethanol extract, while there was significant difference between control sample and the sample fortified with 2% dry. That indicates its better sweet taste which can be taken on its own or in a herbal combination [7].
There were statistical significant differences between all fortified groups and control sample except the sample fortified with 1% dry lion foot leaves powder which recorded no significant difference compared with control sample.
Fortification with lion foot leaves powder and its extracts of water and ethanol on acceptability are shown also in table (5). It was found that, the best result was in the samples fortified with 50ppm ethanol extract, (50ppm and 100ppm) water extract and 1% dry that showed no significant difference between them and control sample. While, there were statistical significant differences between control sample and 100ppm ethanol extract also, 2% dry. The sample fortifies with 2% dry recorded the lowest value in all fortified samples.
References
- Falchero L, Coppa M, Fossi A, Lombardi G, Ramella D, Tava A. Essential oil composition of lady's mantle (Alchemilla xanthochlora Rothm.) growing wild in Alpine pastures. Nat Prod Res. 2009;23(15):1367-72. doi: 10.1080/14786410802361438. PubMed PMID: 19809907.
- Kaya B, Menemen Y, Saltan FZ. Flavonoid compounds identified in Alchemilla L. species collected in the north-eastern Black Sea region of Turkey. Afr J Tradit Complement Altern Med. 2012 Apr 2;9(3):418-25. PubMed PMID: 23983376.
- Mills S, Bone K. Principles and practice of phytotherapy. Modern herbal medicine. Churchill Livingstone; 2000.
- El-Hadidy EM, Refat OG, Halaby MS, Elmetwaly EM, Omar AA. Effect of Lion’s Foot (Alchemilla vulgaris) on Liver and Renal Functions in Rats Induced by CCl4. Food Sci Nutr. 2018 Jan 17;9(01):46.
- Mradu G, Saumyakanti S, Sohini M, Arup M. HPLC profiles of standard phenolic compounds present in medicinal plants. J Pharmacogn Phytochem. 2012;4(3):162-7.
- Gruenwald J, Brendler T, Jaenicke C. Physician's Desk Reference (PDR) for HerbalMedicines. 3rd edition. Thomson PDR, Montvale; 2004. p. 497.
- Clair Sandra.Traditional Herbal Medicine Lady’s Mantle: A Woman’s Best Friend. Journal of the New Zealand Association of Medical Herbalists. 2009.
- Ahmad D, Gulfraz M, Ahmad MS, Nazir H, Gul H, Asif S. Protective action of Taraxacum officinale on CCl4 induced hepatotoxicity in rats. Afr J Pharm Pharmacol. 2014 Aug 15;8(30):775-80.
- Chandrasena LG, Chackrewarthy S, Perera PT, de Silva D. Erythrocyte antioxidant enzymes in patients with cataract. Ann Clin Lab Sci. 2006 Spring;36(2):201-4. PubMed PMID: 16682518.
- Murugesan GS, Sathishkumar M, Jayabalan R, Binupriya AR, Swaminathan K, Yun SE. Hepatoprotective and curative properties of Kombucha tea against carbon tetrachloride-induced toxicity. J Microbiol Biotechnol. 2009 Apr;19(4):397-402. PubMed PMID: 19420997.
- Onakpoya I, Spencer E, Heneghan C, Thompson M. The effect of green tea on blood pressure and lipid profile: a systematic review and meta-analysis of randomized clinical trials. Nutr Metab Cardiovasc Dis. 2014 Aug;24(8):823-36. doi: 10.1016/j.numecd.2014.01.016. PubMed PMID: 24675010.
- Girish C, Pradhan SC. Hepatoprotective activities of picroliv, curcumin, and ellagic acid compared to silymarin on carbon-tetrachloride-induced liver toxicity in mice. J Pharmacol Pharmacother. 2012 Apr;3(2):149-55. doi: 10.4103/0976-500X.95515. PubMed PMID: 22629090.
- Slinkard K, Singleton VL. Total phenol analysis: automation and comparison with manual methods. Am J Enol Vitic. 1977 Jan 1;28(1):49-55.
- Ordonez AA, Gomez JD, Vattuone MA. Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chem. 2006 Aug 1;97(3):452-8.
- Taira S. Astringency in persimmon. InFruit analysis. Springer, Berlin: Heidelberg; 1996. p.97-110.
- Hiai S, Oura H, Nakajima T. Color reaction of some sapogenins and saponins with vanillin and sulfuric acid. Planta Med. 1976 Mar;29(2):116-22. PubMed PMID: 948509.
- Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995 Jan;43(1):27-32.
- Reeves PG, Nielsen FH, Fahey GC Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993 Nov;123(11):1939-51. PubMed PMID: 8229312.
- Campbell JA. Methodology of protein evaluation RAG Nutr. Document R. 1963;10.
- Jayasekhar P, Mohanan PV, Rathinam K. Hepatoprotective activity of ethyl acetate extract of Acacia catechu. Indian J Pharmacol. 1997 Nov 1;29(6):426-8.
- Young DS, Friedman RB. Effects of disease on clinical laboratory tests. Amer Assn for Clinical Chemistry; 2001.
- Ranganna S. Handbook of analysis and quality control for fruit and vegetable products. Tata McGraw-Hill Education; 1986.
- European Pharmacopoeia. Alchemillae Herba. 2008;6:2.
- Jayathilake C, Rizliya V, Liyanage R. Antioxidant and free radical scavenging capacity of extensively used medicinal plants in Sri Lanka. Procedia Food Sci. 2016 Jan 1;6:123-6.