Spoilage Potentials of Biodeteriogens on Two Edible Oils - Groundnut (Arachis Hypogaea) and Palm (Elaeis Guineensis) Oil Sold in Rumuowoji Ultra - Modern Market, Port Harcourt, Nigeria
Odili Chukwudi1, Anthony A. Ibiene1, Dinebari P. Berebon2*, Christopher O. Eze2, Ezinnwanne C. Ezeibe2, Anthony I. Onah2
1 Department of Microbiology, Faculty of Science, University of Port Harcourt, P.M.B. 5323, Choba, Port Harcourt, Nigeria.
2 Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria.
*Corresponding Author
Dinebari P. Berebon,
Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, University of Nigeria, Nsukka, 410001, Enugu State, Nigeria.
Tel: 08064696374
E-mail: dinebari.berebon@unn.edu.ng
Received: July 14, 2020; Accepted: August 04, 2020; Published: August 06, 2020
Citation: Odili Chukwudi, Anthony A. Ibiene, Dinebari P. Berebon, Christopher O. Eze, Ezinnwanne C. Ezeibe, Anthony I. Onah. Spoilage Potentials of Biodeteriogens on Two Edible Oils - Groundnut (Arachis Hypogaea) and Palm (Elaeis Guineensis) Oil Sold in Rumuowoji Ultra - Modern Market, Port Harcourt, Nigeria. Int J Food Sci Nutr Diet. 2020;9(5):470-476. doi: dx.doi.org/10.19070/2326-3350-2000083
Copyright: Dinebari P. Berebon© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
The spoilage potential and physicochemical characteristics of biodeteriogens on edible oils (groundnut oil and palm oil) sold in
the market (Rumuowoji ultra-modern market) within Port Harcourt were examined. Spoilage bacterial genera were identified as
Bacillus spp, Pseudomonas spp, Micrococcus spp and Staphylococcus spp while the fungal genera were identified as Aspergillus
spp, Penicillium spp and Fusarium spp. The screen test for the utilization of the oil samples by the biodeteriogens showed that 7
out of the 9 bacterial species and 4 out of the 5 fungal species were capable of utilizing the edible oils as sole source of carbon
and energy. Utilization of both oils by the mixed bacterial cultures of Bacillus spp. A1, Pseudomonas spp. A2, Bacillus spp. B1 and
Staphylococcus spp. B3 resulted in maximum increases in total viable counts (TVC) of 1.5 x 108 cfu/ml as optical density (OD)
increases to 1.31 nm while pH decreases with time from 7.10 - 5.65 over a 20 days’ incubation. Similarly, the TVC, OD and pH
of the mixed fungal culture of Aspergillus spp Af3, Aspergillus spp Bf1 and Penicillium spp Bf2 on palm oil and groundnut oil where:
1.09 x 106 cfu/ml, 1.01 nm; 7.0 – 6.0 and 0.9 x 105 cfu/ml, 0.81nm, 7.2 - 6.1 respectively. The physiochemical analyses of the
palm oil samples revealed that the highest mean free fatty acid (FFA) content and the peroxide value (PV) were 5.08% and 7.98
mEq/kg respectively in palm oil samples inoculated with mixed bacterial culture.
2.Introduction
3.Materials And Methods
4.Results And Discussions
5.Conclusion
6.References
Keywords
Biodeteriogens; Edible Oils; Spoilage and Physicochemical Characteristics.
Introduction
Edible oil is not a sterile product. The micro flora of edible oil
reflects the quality of oil, the sanitary conditions of processing
equipment used to manufacture the oil as well as the environmental
and sanitary conditions during packaging and handling of such
product [1]. Fat and oil which are naturally occurring constituents
of edible oil are subject to microbial deterioration. Essential fatty
acids (EFAs) which are naturally occurring constituents of edible
oil have been considered as functional food and nutraceuticals.
Beside their use in the production of soap and other surface-active
molecules, fat and oil are increasingly used as a source of fuel
energy producing purposes in automobiles, trains, aeroplanes or
boats, or the direct production of biodiesel [2]. These new uses
underlie the food versus fuel debate of edible oil [3].
Both groundnut oil and palm oil are the primary sources of edible
oils used in most household in Nigeria. Currently, Nigeria is the
fifth world leading producers of palm oil after Columbia, Thailand,
Malaysia and Indonesia being the first [4]. Unlike groundnut
oil, palm oil is rich in palmitic acid with a reddish brown color
impacted by ß - carotene. Edible fats and oils usually referred to as
lipids contain 95-98% triacylglycerols, lesser percentages of diacyl-
and mono-acylglycerols, free fatty acids, and 1–2% of nonsaponifiable
components, such as sterols, tocopherols, and color
compounds [5].
Edible oils are known to support the growth of bacteria, fungi
and molds especially when they contain moisture. The composition
of these oils determines the extent and type of organisms
likely to thrive in them. These organisms cause chemical changes
that lead to deterioration in the quality of the oils.
Although several researchers have reported the quality assessment of palm oil produce marketed within some states of Nigeria:
Abia State [6], Anambra State [7], Delta State [8], Ibadan [9],
Kogi State [10] and Rivers State [11]; such report indeed represented
a fractional totality of the quality assessment of palm oil
sold in such states. It is thus pertinent to further investigate other
unexplored or un-sampled regions within the states to provide a
more accurate baseline data on the quality of palm oil on sale in
its markets. Thus, this study is aimed at examining the potential
biological contaminants (bacteria and fungi) contaminating edible
oils (groundnut oil and palm oil) sold in the market (Rumuwoji
Ultra-modern market) within Port Harcourt as well as their physicochemical
characteristics.
Samples of the edible oils (palm oil and groundnut oil) were obtained
from Rumuwoji Ultra-Modern Market (Mile One), Port
Harcourt. The samples were collected in sterile universal bottles.
Care was taken not to contaminate the bottles before and during
collection of the samples. The samples were taken to the laboratory
for microbiological and physicochemical analyses.
Enrichment and pour plate technique was adopted for the isolation
of bioteriogens in the oil samples. Five drops of each type of
oil were added to 10 ml of nutrient broth and incubated at room
temperature for 48 h. Following this enrichment, a 10-fold serial
dilution of the culture was performed using sterile physiological
saline as diluent and 100 μl of appropriate dilutions plated on
nutrient agar and potato dextrose agar (PDA). Nutrient agar and
PDA were each fortified with 50 mg/l of nystatin and chloramphenicol
to inhibit the growth of fungi and bacteria respectively.
Pour plate method was adopted for isolation. Nutrient agar plates
and PDA plates were incubated at room temperature 37ºC for
24-48 h and 25ºC for 3-5 days respectively. The bacterial colonies
which developed on the nutrient agar plates were picked and subcultured
on fresh nutrient agar plates using the streak plate technique
for discrete colonies of bacteria. The fungal colonies which
developed on the PDA plates were also picked and sub-cultured
on fresh PDA plates using a sterile inoculating needle for discrete
colonies of fungi.
The bacterial isolates were characterized and identified based on
their morphological and biochemical characteristics. Gram staining,
spore stain, oxidase test, catalase test, coagulase test, methyl
red and Voges-Proskauer (MRVP) test, indole test, citrate test,
motility test, sugar fermentation test and triple sugar iron agar
(TSIA) test were all carried using standard microbiological procedures.
Macroscopic and Microscopic examination were used for
the characterization and identification of fungal isolates. The later
was done by needle mount method.
The organisms (bacteria and fungi) isolated from an oil (groundnut
oil or palm oil) were tested for utilization of the oil using a modified mineral salts medium [12] containing the oil as sole
source of carbon and energy. The medium was dispensed in 9.9
ml amounts in test tubes. To half of these tubes were added 0.1ml
each of groundnut oil and to the remaining tubes were added 0.1
ml each of palm oil. After capping, all the tubes were sterilized by
autoclaving at 121ºC for 15 minutes and allowed to cool. Upon
cooling, each set of tubes were inoculated with an isolate from
the oil. Two control tubes (one containing groundnut oil and the
other palm oil) remained uninoculated. All the tubes were incubated
at room temperature for 14 days. Each tube was checked for
turbidity after the incubation period.
A modified mineral salts medium [12] was prepared and dispensed
in 99 ml quantities into 250 ml Erlenmeyer flasks. To each
of these flasks were added 1 ml each of fresh oil. The mixture was
autoclaved at 121ºC for 15 minutes. Isolates that showed highest
turbidity in the screen test were used as mixed cultures to inoculate
flasks containing that oil. Two flasks (one containing groundnut
oil and the other palm oil) remained uninoculated and served
as controls. The flasks were incubated at room temperature on
a rotary shaker incubator (Staurt orbital incubator, S150, OSA,
UK) operated at 120 rpm for 20 days. The optical density (OD) at
560 nm, total viable counts (TVC) and pH of the culture in each
flask were monitored periodically at intervals of 4 days. The optical
density (OD) at 560 nm was measured using spectrophotometer
(spectrumlab 7525). Total viable counts were obtained from
nutrient agar plates and potato dextrose agar plates for bacteria
and fungi respectively after serial dilution in sterile physiological
saline. The pH of the culture was measured using a pH meter
equipped with glass electrode.
The remaining oils in the mineral salt medium at the end of the
incubation period were extracted in order to determine the extent
of biodeterioration by the isolates. The extraction was performed
by adding 10 ml of thoroughly shaken medium containing the
residue of the oil to a separating funnel. To this was added 10ml
of n-heptane. The funnel was vigorously shaken, and the contents
were allowed to settle in order for the phases to separate. Upon
separation, the solvent layer was drawn off into a clean glass vial.
The aqueous phase was further mixed with 5ml fresh solvent and
the extraction procedure was repeated. The two extracts were
pooled and stored in the refrigerator (4ºC) until ready for analysis.
The extracts were then analyzed using a computerized gas chromatography
equipped with a flame ionization detector (FID).
The free fatty acid (FFA) content and peroxide value (PV) of the
unionoculated and inoculated oil samples were determined according
to the methods described [13].
All data were represented are means of duplicates and values subjected
to statistical analysis using SPSS 20. Data were represented
with graphs and tables.
Results And Discussions
Biodeteriogens of edible oil are consortia of microorganisms
which cause spoilage while utilizing the oil or its constituents as
a substrate - source of carbon and energy. The results indicated
that bacteria, fungi and molds are the potential biodeteriogens
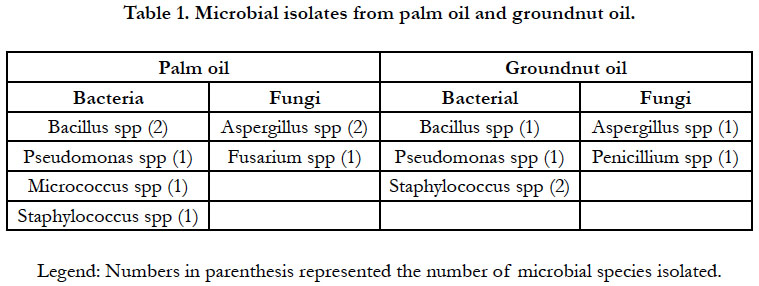
of edible oil. A summary of the microbial isolates from palm oil
and groundnut oil is presented in Table 1. The bacteria identified
were members of the following genera: Bacillus spp, Pseudomonas
spp, Micrococcus spp and Staphylococcus spp while fungal genera identified
were Aspergillus spp, Penicillium spp and Fusarium spp. These
bacteriogens have previously been implicated in spoilage of vegetable
oils and palm oil sold within Amassoma, Bayelsa State [14],
Jos Metropolis [15, 16]. Interestingly, Bacillus, Pseudomonas and
Staphylococcus were found to contaminate both oils. Micrococcus
was found to contaminate palm oil only. It was observed from
this study that the predominant bacterial flora were Gram positive
bacteria. Some of these bacteria are pathogenic and can cause
food poisoning. Their presence in the edible oils may be due to
chance contamination during handling or storage and biofouling
of processing equipment in contact with the oil.
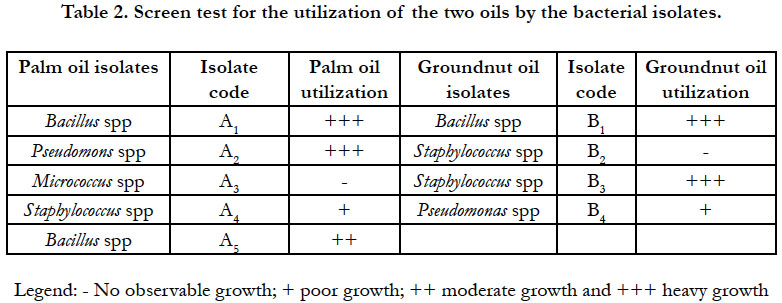
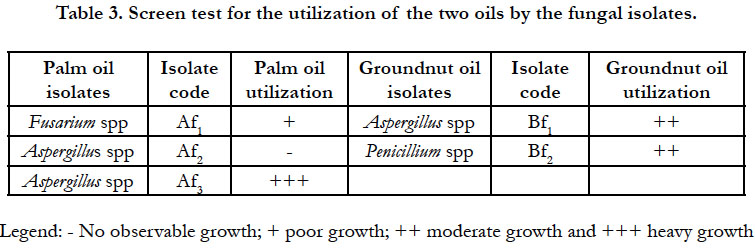
The results of the screen test for the utilization of the two edible oils as sole source of carbon and energy by the bacterial and fungal isolates are presented in Table 2 and 3 respectively. Some of the isolates did not produce turbidity in the mineral salt broth cultures. Those cultures that did not show any turbidity were restreaked out on nutrient agar plates and PDA plates for bacteria and fungi respectively to check for viability. Growth occurred in all the plates inoculated indicating that the organisms were still viable but could not utilize the oils as sole carbon and energy sources for growth. Five out of the 9 bacterial species and 4 out of the 5 fungal species were capable of growing and utilizing the edible oils as sole source of carbon and energy. This suggests that the remaining species were merely chance contaminants and might not have significant spoilage activity on the oils.
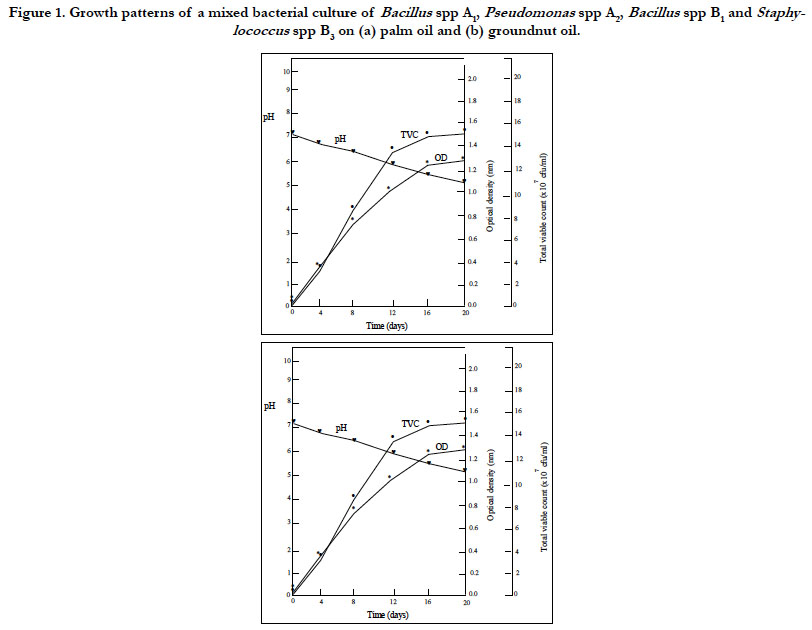
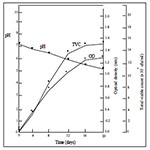
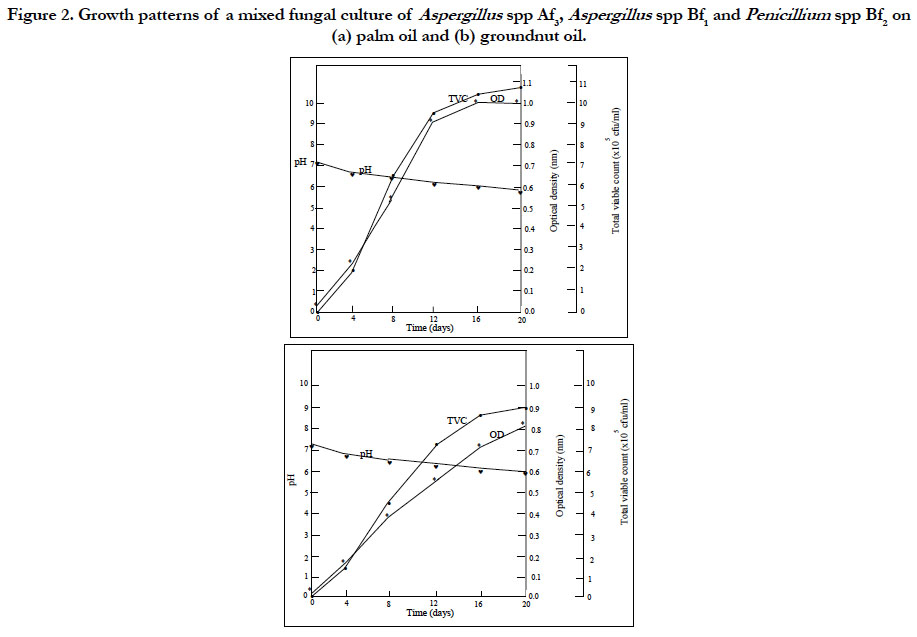
Figures 1 and 2 shows the result of the growth patterns of the mixed cultures determined by monitoring the optical density (OD), total viable counts (TVC) and pH of the cultures utilizing the two oils as sole carbon and energy sources. The cultures showed that as the TVC increased, there were corresponding increases in OD and decreases in pH with time. The utilization of the edible oils as sole source of carbon and energy by the organisms resulted in their growth with a concomitant production of acidic metabolites. These acidic metabolic products might account for the decreases in pH of the cultures. Organoleptic changes and increase in acidity may result when oils are metabolized by microorganisms. It was further observed in Figures 1 and 2 that the cultures had reached the stationary phase of growth. This observation is attributed to the rate of utilization of the two oils by the microorganisms and the stability of these oils to microbial spoilage. The results showed that palm oil supported the highest microbial cell growth while groundnut oil supported the least cell growth. These results indicated that groundnut oil was the most stable to microbial spoilage while palm oil was the least biostable.
Figure 1. Growth patterns of a mixed bacterial culture of Bacillus spp A1, Pseudomonas spp A2, Bacillus spp B1 and Staphylococcus spp B3 on (a) palm oil and (b) groundnut oil.
Figure 2. Growth patterns of a mixed fungal culture of Aspergillus spp Af3, Aspergillus spp Bf1 and Penicillium spp Bf2 on (a) palm oil and (b) groundnut oil.
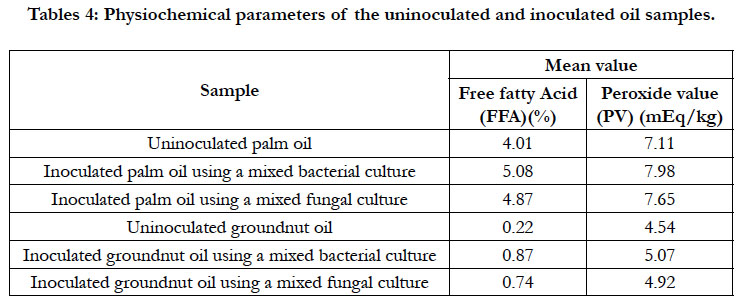
The results of the free fatty acid (FFA) content ranged from 0.22 - 5.08 % in the uninoculated groundnut oil and inoculated palm oil samples as presented in Table 4. The result showed that there was an increase in FFAs content of the oils at the end of the incubation period. High concentrations of FFAs are undesirable in edible oils as it reduces the palatability and the shelf-life of the oil [17]. This elevation of FFAs is an indication of contamination of the oil by the biodeteriogens. Palm oil inoculated with a mixed bacterial culture has the greatest FFA increase of 5.08 % followed by palm oil inoculated with a mixed fungal culture with a FFA increase of 4.87 %. Groundnut oil inoculated with a mixed bacterial culture had a FFA increase of 0.87%. Groundnut oil inoculated with a mixed fungal culture has the least free fatty acid increase of 0.74 %. This results corroborated with other findings [18] who observed that palm oil marketed in Ibadan is hard as their FFAs exceeded 5% as well as greater than the reported 2.86 to 2.97 mg/g, 2.73 to 2.89 mgKOH/g, 2.67 to 4.20 % and 2.73 to 2.83 mg KOH/g obtained in palm oil samples from Delta State, Jos metropolis in Plateau State, Ihiala Local Government of Anambra State and Abia State as reported [6, 7, 8, 16], respectively.
These differences in the biochemical pattern of the organisms on the oils may be related to the differences in the fatty acid composition of the oils. Fatty acids play a very important role in fats and oils because of their health implications in the human diet and properties in industrial processes. The type of fatty acid determines the nutritional status and storability (keeping quality) of the oil. The major fatty acids predominant in palm oil being oleic and palmitic acids. Palmitic acid increases in palm oil are mostly associated with oils produced from over ripe, bruised and crushed fruits, fruits subjected to severe impacts from loading and off-loading bunches and oils stored over long periods [19-21]. Groundnut oil has a high proportion of oleic acid [22]. The palm oil sample (inoculated and uninoculated) has the greatest FFA and account for why it was the least biostable.
The PV of the groundnut oil inoculated with mixed fungal culture and palm oil with a mixed bacterial culture ranged from 4.92 - 7.98 mEq/kg as shown in Table 4. This PV ranges are acceptable since they are lower related to the standard value of 10 mEq/kg for vegetable oil deterioration as specified by the standard guidelines set by NAFDAC and CODEX [23-25]. Table 4 further shows that at the end of the incubation period, there was an increase in the peroxide value of the inoculated oils. The PV is an index that measures the degree to which the oil has undergone rancidity or oxidation indicative of microbial growth. Palm oil samples obtained from red and yellow fruits of the dura variety had higher perioxide value due to microbial growth [26]. The peroxide value determines the degree of oxidation of oil as well as gives an indication of the level of deterioration of oils and fats [27]. It was earlier observed that lipolytic fungi while contributing to the deterioration of vegetable oil by increasing the amount of free fatty acid, do not effect the oxidation state of the oil [22]. This observation might be responsible for the low peroxide value increase recorded in groundnut oil using a mixed fungal culture in this study.
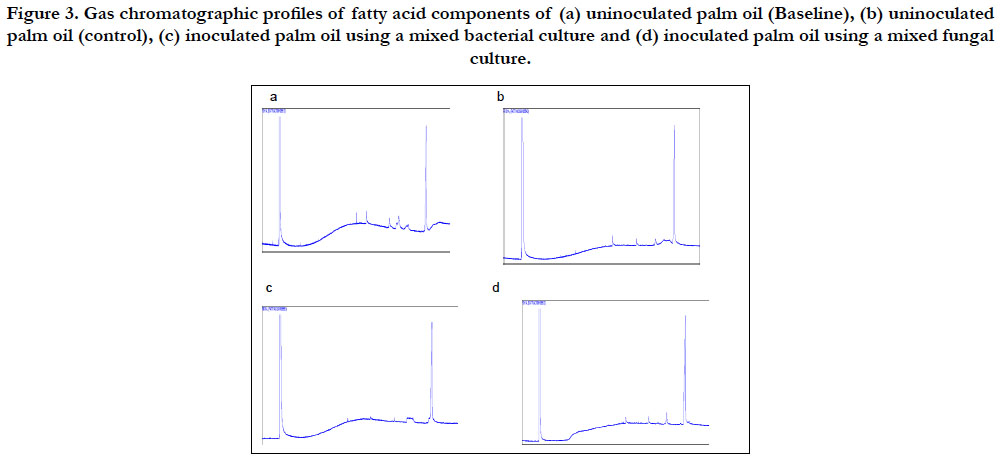
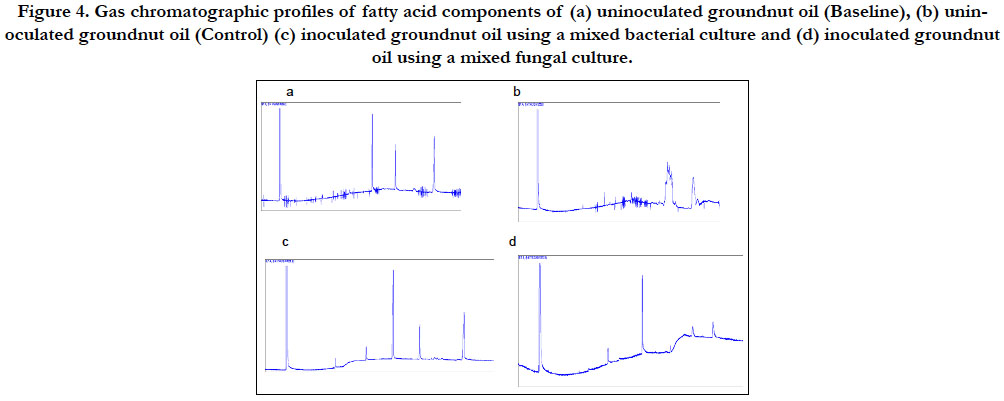
The chromatographic tracings of the methylated heptane extracts of the two oils are presented in Figures 3 and 4. The figures revealed that the inoculated oils showed a reduction in the sizes of peaks when compared to their uninoculated oils. The reduction recorded in the sizes of the peaks is a clear indication that the components of the oils were utilized and degraded by the isolates.
Figure 3. Gas chromatographic profiles of fatty acid components of (a) uninoculated palm oil (Baseline), (b) uninoculated palm oil (control), (c) inoculated palm oil using a mixed bacterial culture and (d) inoculated palm oil using a mixed fungal culture.
Figure 4. Gas chromatographic profiles of fatty acid components of (a) uninoculated groundnut oil (Baseline), (b) uninoculated groundnut oil (Control) (c) inoculated groundnut oil using a mixed bacterial culture and (d) inoculated groundnut oil using a mixed fungal culture.
Bacteria were the predominant organisms isolated from this study. There was preponderance of bacteria over fungi in this study. Some of the microorganisms isolated can cause health problems in individuals who consume the product without heat processing. Some are food spoilage organisms and may accelerate the deterioration of the oils.
Some Bacillus species are pathogenic and can cause food poisoning. Their presence in the oil samples may be due to the exposure of the oil sample to the spores of the organism which are dormant and are highly resistant to the lethal effects of heat drying and ultraviolet radiation. Bacillus species has versatile intrinsic deterioration potentials due to production of protease and lipases that are stable at high temperature, at alkaline pH and in the presence of detergents, tolerance to high pH concentrations, oil and grease as well as biofilms formation potentials. Some species of Staphylococcus are capable of producing enterotoxin and are noted to survive for extended periods in hostile environments. They could cause gastroenteritis in the individual if the oil is consumed raw. Their isolation could be an indication of unhygienic handling of the oils by the sellers or contamination during production cycle.
Species of Aspergillus, Penicillium and Fusarium are of health significance because they are known to produce toxin. These fungi produce a group of toxic metabolites known as aflatoxin which is capable of inducing toxic syndromes especially cancer. Since deteriorated edible oils pose certain health risk, there is need to prevent factors that could cause their deterioration.
This study revealed that palm oil was the least biostable to microbial deterioration while groundnut oil was the most biostable. However, this can be attributed to the differences in the intrinsic and extrinsic requirements of microorganisms and hence microbial utilization of the constituents of the oil.
Conclusion
Microbial deterioration of edible oils is greatly dependent on the
microbial species capable of utilizing the fatty acid components
of the oils as their sole source of carbon and energy. Palm oil
was found to contain the highest number of microbial species
and supported the highest cell growth compared to groundnut oil.
The presence of the microbial species in the oil samples may be
due to contamination during harvesting, processing, handling and
storage. The scale of operations differs and this affects the quality
of the final product. Apart from fouling the organoleptic properties
of the edible oil, these biodeteriogens ferment components
of the oil to produce toxic chemicals or toxins detrimental to human
consumption if not evaluated for microbial quality.
References
- World health organization. Food safety and food borne diseases and value chain management for food safety. “Forging links between agriculture and health” CGIAR on agriculture and health meeting in WHO/HQ; 2007.
- Gunstone FD. Composition and properties of edible oils. Edible oil process-ing. 2000:1-33.
- Gunstone FD. The food-fuel debate. Vegetable Oils In Food Technology. 2nd edn. Oxford, UK. 2011; 2: 19-21.
- Sawe BE. Top Palm Oil Producing Countries In The World. Retrieved August. 2018; 19: 2019.
- Kochhar P. Thermal Stability of Fats for High Temperature Applications. Functional Dietary Lipids. Woodhead Publishing. 2016; 103–148.
- Udensi EA, Iroegbu FC. Quality assessment of palm oil sold in major markets in Abia State, Nigeria. Agroscience. 2007; 6(2): 25-27.
- Okonkwo SI, Ogbuneke RU. Assessment of level of adulteration in palm oil (Elaeis guineensis) within Ihiala Local Government Area of Anambra State of Nigeria. J. Basic Phys. Res. 2010; 1: 13-16.
- Agbaire PO. Quality assessment of palm oil sold in some major markets in Delta State, Southern Nigeria. Afr. J. Food Sci. Technol. 2012; 3(9): 223- 226.
- Olorunfemi MF, Oyebanji AO, Awoite TM, Agboola AA, Oyelakin MO, Alimi JP, et al. Quality assessment of palm oil on sale in major markets of Ibadan, Nigeria. IJFR 1. 2014; 1: 8-15.
- Enemuor SC, Adegoke SA, Haruna AO, Oguntibeju OO. Environmental and fungal contamination of palm oil sold in Anyigba Market, Nigeria. Afr. J. Microbiol. Res. 2012; 6(11): 2744-2747.
- Ohimain EI, Daokoru-Olukole C, Izah SC, Alaka EE. Assessment of the quality of crude palm oil produced by smallholder processors in Rivers State, Nigeria. Nigerian J. Agric. Food Environ. 2012; 8(2): 28-34.
- Bharathi P, Elavarasi N, Mohansundaram S. Studies on rate of biodegradation of vegetable (coconut) oil by using Pseudomonas aeruginosa. International Journal of Environmental Biology. 2012.
- Pearson D .The Chemical Analysis of Food. 8th edn. JA Churchill, London. 1981: p- 535.
- Seiyaboh EI, Kigigha LT, Alagoa CT, Izah SC. Microbial Quality of Palm Oil Sold in Amassoma, Bayelsa State, Nigeria. Int J Pub Health Safe. 2018; 3: 153.
- Okechalu JN, Dashen MM, Lar PM, Okechalu B and Gushop T. Microbiological quality and chemical characteristics of palm oil sold within Jos Metropolis, Plateau State, Nigeria. J. Microbiol. Biotechn. Res. 2011; 1(2): 107-112.
- Odoh CK, Tarfen YA, Orjiakor IP, Martins PE, Seibai BT, Akpi UK, et al. Assessment of mold contamination and physicochemical properties of crude palm oil sold in Jos, Nigeria. Food Science & Nutrition published by Wiley Periodicals, Inc. 2016; 5(2): 310-316. PMID: 28265365.
- Babatunde OA, Bello GS. Comparative Assessment of Some Physicochemical Properties of Groundnut and Palm Oils Sold Within Kaduna Metropolis, Nigeria. IOSR Journal of Applied Chemistry. 2016; 9(11): 2278-5736.
- Olorunfemi MF, Oyebanji AO, Awoite TM, Agboola AA, Oyelakin MO, et al. Quality assessment of palm oil on sale in major markets of Ibadan, Nigeria. IJFR. 2014; 8-15.
- Salunkhe DK, Chavan JK, Adsule RN, Kadam SS. World oil Seeds. Chemistry, Technology and utilization. Van Nostrand Reinhold, New York. 1992; 54.
- Tagoe SMA. Effect of mycotoxigenic microorganisms on palm fruits and palm oil produced at the cottage industry level. Nottingham University, United Kingdom, PHD Thesis. 2008.
- Tagoe SMA, Dickson MJ, Apetorgbor MM. Factors influencing quality of palm oil produced at the cottage industry level in Ghana. International food Research Journal. 2012; 19(1):271-278.
- Molokwu CN, Okpokwasili GC. Biodeterioration potential of fungi isolates from vegetable oils. International Journal of food science and Nutrition. 1997; 48(4): 251-255.
- SON. Standard Organization of Nigeria. Standards for Edible Refined Palm Oil and Its Processed form. 2000; 2–5.
- NIS. Nigerian Industrial Standards. Standard for Edible Vegetable Oil. 1992; 5–12.
- Alimentarius C. Codex general standard for food additives. Codex standard. 1995:192-95.
- Ekpa OD, Ekpe UJ. Effect of Coconut Oil Concentration on the Melting point profile and Free Fatty Acid Formation of Palm Oil. Nig. J. Chem. Res. 1996; 1: 8-12.
- Ekwenye UN. Chemical characteristics of palm oil bioderioration. Chemical Society for Experimental Biology. 2005; 18: 141-149.