Estrogen Ameliorates Photoreceptor Cell Loss In Light-Induced Retinal Degenerated Balb/C Mice
Bandyopadhyay M1,2*, O'Quinn E2, Seidman L2, Rohrer B2*
1 Department of Biological Sciences, Trident Technical College, North Charleston, SC, USA.
2 Department of Ophthalmology, Medical University of South Carolina, Charleston, SC, USA.
*Corresponding Author
Mausumi Bandyopadhyay Ph.D,
Department of Biological Sciences, Trident Technical College,
North Charleston, South Carolina 29206, USA.
Tel: 843-754-7371
Fax: 843-574-6751
E-mail: mausumi.bandyopadhyay@tridenttech.edu
Bärbel Rohrer, Ph.D,
Department of Ophthalmology,
Medical University of South Carolina, Charleston, SC 29425, USA.
Received:August 16, 2017; Accepted: September 21, 2017; Published: October 03, 2017
Citation: Bandyopadhyay M, O'Quinn E, Seidman L, Rohrer B. Estrogen Ameliorates Photoreceptor Cell Loss In Light-Induced Retinal Degenerated Balb/C Mice. Int J Ophthalmol Eye Res. 2017;5(8):341-347. doi: dx.doi.org/10.19070/2332-290X-1700069
Copyright: Bandyopadhyay M, Rohrer B© 2017. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Purpose: Estrogen or 17β-estradiol (17β-E2) is known to act as an antioxidant and has neuroprotective effects. The purpose of this study is to evaluate the effects of estrogen and estrogen receptor beta (ERβ) on photoreceptor cell structure and function in an experimental model for light-induced photoreceptor degeneration.
Methods: 3-5 months old Balb/c mice were exposed to constant light of ~1500 lux. Mice were divided into four groups and treated via intraperitoneal injections with vehicle, 17β-E2, the ERβ inhibitor [4-(2-phenyl-5,7-bis(trifluoromethyl) pyrazolo[1,5-a]pyrimidin-3-yl)phenol, (PHTPP), or a combination of 17β-E2 and PHTPP. Retinal function was evaluated by electroretinogram (ERG), and eyes were assessed by histology and immunohistochemistry.
Results: Light damage resulted in a loss of the number of photoreceptor nuclei in the outer nuclear layer (ONL), a loss of UV cone opsin immunoreactivity and a reduction in rod- and cone-mediated ERG signals. 17β-E2 treatment significantly increased the ONL cell count and increased the rod-driven a-wave in the ERG. PHTPP alone had no effect, but PHTPP eliminated the protective effect of exogenously applied estrogen. Likewise, 17β-E2 significantly increased the amount of UV cone opsin immunoreactivity, an effect that was negated by co-administration of PHTPP.
Conclusions: Estrogen supplementation was found to reduce rod and cone photoreceptor cell damage in this light-induced photoreceptor degeneration mouse model, an effect that was mediated via ERβ activation.
2.Introduction
3.Materials and Methods
3.1 Animals
3.2 Light Damage
3.3 Estrogen and Estrogen Inhibitor Treatment
3.4 Electroretinography (ERG)
3.5 Histology
3.6 Immunohistochemistry
4.Results
4.1 Estrogen Treatment Decreases Rod Photoreceptor Cell Death in Light Damaged BALB/c Mice
4.2 Effect of Estrogen on Light Induced Photoreceptor Cell Damage in BALB/c Mice were Mediated via ERβ
4.3 Estrogen Reduces Light-Induced Loss of Rod and Cone Photoreceptor Cell Function
4.4 Estrogen Prevents Light-Induced UV Cone Opsin Loss
5.Discussion
6.Conclusion
7.Acknowledgements
8.References
Keywords
Light Damage; Photoreceptor; Estrogen (17β estradiol); [4-(2-phenyl-5,7-bis(trifluoromethyl)pyrazolo[1,5-a] pyrimidin-3-yl)phenol(PHTPP); Estrogen Receptor Beta.
Introduction
Photoreceptor cells in the retina are capable of absorbing light and thus initiate a photo response. There are two types of photoreceptors; rods and cones. Rods are extremely sensitive to light, whereas cones require significantly more light to trigger a photo-response. Light, an environmental factor, has been shown to cause the death of a significant amount of rod photoreceptors when exposed continuously for a certain period of time [1, 2]. Hao and colleagues [3] have shown using mice in which essential components of the photoreceptor signal transduction cascade were eliminated, that there exist two different pathways of light-induced photoreceptor cell death; bright light leads to photoreceptor cell-loss that is independent of transducin; whereas low light induces a pathway that requires transducin. Organisciak and Vaughan have revealed the oxidative stress mediated apoptosis in photoreceptor cells after a continuous light exposure [4-6]. Oxidative stress is a potent contributor to age related macular degeneration (AMD), particularly dry AMD involving atrophy to the retinal pigment epithelium (RPE) followed by the slow degeneration and subsequent photoreceptor cell loss.
Light-induced retinal degeneration is widely accepted to study the mechanism of retinal degeneration and has been used to find out therapeutic efficacy of several bio molecules in photoreceptor degeneration [4, 6, 7]. We examined the efficacy of 17 beta-estradiol (17β-E2) in the light induced retinal degeneration model.
Estrogen has protective function. The protective efficacy of estrogen has been found in a variety of tissues [8]. Different investigators have shown that 17 beta-estradiol protects RPE from oxidative stress [9-11]. Additionally, reports also suggest that 17β-E2 pretreatment reduces RPE degeneration by up regulating the apoptosis related proteins [11]. Furthermore, estrogen has a versatile therapeutic effect, ranging from immunosuppression and anti-inflammatory mode of action to facilitating regeneration of neurons in brain. Moreover, estrogen deficiency has been found to increase sub-RPE deposits in high-fat fed C57BL/6 mice [9, 12]. Also, females are particularly susceptible to retinal degeneration after menopause, and those who take hormone replacement therapy are at a lower risk [13]. Likewise, older women are more susceptible to develop AMD than men of the same age [9].
Estrogen mediates its functions mainly through its receptors, ER alpha and ER beta on the target tissue [14, 15]. Once the ER is activated by estrogen, ER translocates to the nucleus and acts as hormone inducible transcription factors [16]. Such ER receptors are also found on the retina [17]. Here we evaluated the effects of estrogen on photoreceptor cell structure and function in an experimental model of light-induced photoreceptor degeneration. Our results showed that ten days of constant light exposure caused retinal damage in BalB/c mice model with a reduction in the rows of rod photoreceptor nuclei in the outer nuclear layer of the retina. The extent of such experimentally induced photoreceptor cell damage is lowered significantly by estrogen treatment. The results also demonstrated significant morphological and functional restoration of photoreceptor cells ensuing estrogen treatment. Further research is under way to explore the therapeutic effects of estrogen in retinal disorders.
Balb/c mice were generated from breeding pairs obtained from Harlan Laboratories (Indianapolis, IN). Mice were housed in the Medical University of South Carolina animal care facility under a 12-hour light/12-hour dark cycle with access to food and water ad libitum. The ambient light intensity at the eye level of the mice was 85 ± 18 lux. All experiments were performed in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Institutional Animal Care and Use Committee.
Light exposure experiments were performed on 3 month old Balb/c mice. Such experiments consisted of constant fluorescent illumination of approximately 1500 lux for 10 days, as described previously [18], ensuring that all cages were equidistant to the light source. Furthermore, the control group of mice was kept in the same cages as their experimental littermates.
Balb/c mice were kept in constant light for 10 days. During the light damage period, the first group received a 0.2 mg/kg dosage of 17-beta-estradiol each day while the second group received PHTPP (estrogen inhibitor) treatment at a dose of 25 μg/kg every two days; and the third group received both 17 beta estradiol and PHTPP. The corresponding control groups only received the vehicle. All compounds were administered using intraperitoneal injections.
Baseline and endpoint ERGs were performed on the experimental and control BALB/c mice. ERG recordings were performed as described previously [19]. In short, mice were dark adapted overnight. After dark adaptation, mice were anesthetized using a ketamine xylazine cocktail. Eyes were dilated with one drop of Phenylephrine HCl (2.5%) followed by one drop of atropine sulfate (1%). The body temperature was maintained with a heating pad set at approximately 37°C. A needle ground electrode was placed in the tail and a reference needle electrode in the forehead. The ERG responses were recorded using electrodes attached to contact lenses that were held in place by drops of methylcellulose. ERGs were recorded with the EPIC -2000 system (LKC Technologies, Inc., Gaithersburg, MD), using a Grass strobe-flash stimulus. The Rod cells were tested at filters of -10 dB, -6 dB, and 0 dB. Stimuli to determine overall retinal responsiveness consisted of 10 msec single-flashes at a fixed intensity (2.48 cd–s/m2) under scotopic conditions. The mouse was then light adjusted. The eyes were finally exposed to continuous flashes of light at 0 dB to test the cones. The ERG data were analyzed as follows: a-waves were measured from the baseline to the initial negative voltage and B-waves were measured from the trough of the a-wave to the peak of the positive b- wave.
Eyes were fixed in 4% paraformaldehyde and cryoprotected in 30% sucrose solution. The tissues were then cut into 12-μm cryostat sections. Slides of sections were stained with 0.1% toluidine blue and rows of photoreceptors in the outer nuclear layer were counted in ten different regions of the entire retina [20].
Tissues were fixed and cryoprotected as described in histology. After the slides were washed in phosphate buffered saline (PBS); they were blocked with 10% normal goat serum and 3% bovine serum albumin. The tissues were incubated overnight in blocking solution containing the UV-opsin antibody (generously provided by Jeannie Chen, University of Southern California, Los Angeles, CA), followed by incubation with the appropriate fluorescently-labeled secondary antibody for 4 h (Molecular Probes, Carlsbad, CA). The control experiments included omission of primary antibody and observation of singly labeled slides through the appropriate filter set. Sections were mounted and analyzed by confocal microscopy (Leica, Bannockburn, IL) using identical settings for all slides [20, 21].
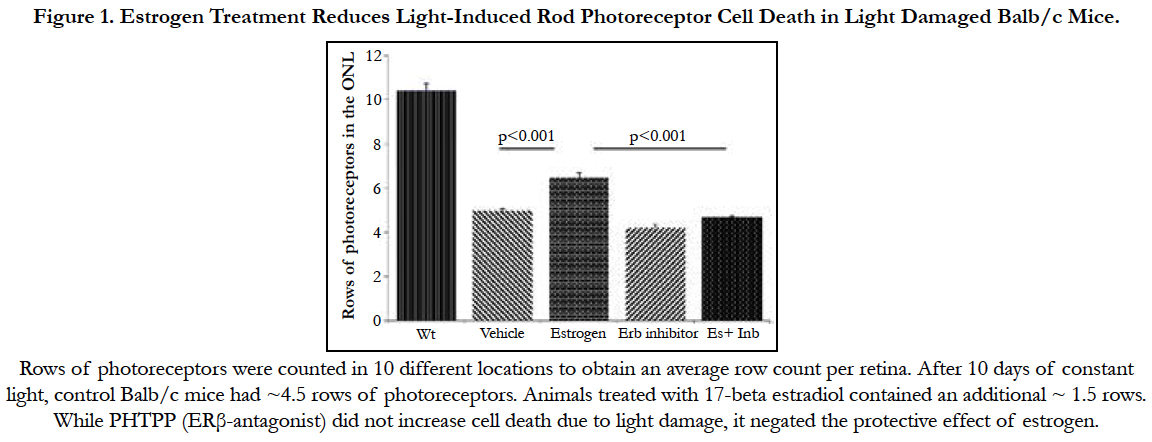
We investigated the effect of estrogen in light damaged Balb/c mice. After 10 days of constant light, control Balb/c mice had ~4.5 rows of photoreceptors. Rows of photoreceptors were counted in 10 different locations to obtain an average row count per retina. Ten days treatment of 17- beta estradiol (0.2mg/kg body weight) caused a reduction in rod photoreceptor cell degeneration (Figure 1) in light damaged Balb/c mice. Animals treated with 17 beta estradiol contained an additional ~ 1.5 rows (P<0.001). (n= 5-10 per condition).
Figure 1. Estrogen Treatment Reduces Light-Induced Rod Photoreceptor Cell Death in Light Damaged Balb/c Mice.
The actions of estrogen are mediated by ERα and ERβ. In order to understand whether the protective effect of estrogen is ERβ mediated, we treated continuously light exposed mice with estrogen receptor beta antagonist, PHTPP, 25μg/kg body weight) every alternate day. Results revealed that estrogen decrease cell death due to light damage (p< 0.01). PHTPP alone did not show any significant change in photoreceptor count. Co-treatment of PHTPP and 17β-estradiol eliminated the protective effect of estrogen (Figure 1).
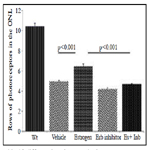
Photoreceptor cell function was determined by using electroretinography (Figure 2). The amplitudes of waveforms were measured to analyze the responses of rods, cones and bipolar cells. Baseline and endpoint ERGs were recorded. ERG responses were analyzed under dark-adapted scotopic conditions to measure rod function. ERG responses to 10 ms single flashes of white light (maximum intensity of 2.48 cd/m2) between 10, 6 and 0 db of attenuation were measured. Light-adapted photopic conditions were used to determine cone function (8 min of constant background light at 7.85 photopic cd/m2; stimulus white light). ERG data was analyzed by comparing the calculated percentage of remaining amplitudes of treatment to control groups.
Figure 2. Estrogen decreases light-induced loss of rod and cone photoreceptor cell function. Photoreceptor cell function was determined using electroretinography. Dark-adapted scotopic conditions were used to measure rod function (3 light intensities using white light; 10, 6 and 0dB of attenuation; max intensity 2.48 photopic cd–s/m2); light-adapted photopic conditions to determine cone function (10 min of constant background light at 7.85 photopic cd/m2; stimulus white light). (A) Mice treated with estrogen showed a significant increase in rod photoreceptor function. (B) PHTPP by itself did not lead to further deterioration of function; but blunted the protective effects of estrogen when co-administered (D). Mice treated with estrogen also showed about 20% increase in cone photoreceptor cell function (2C) but co-treatment with PHTPP eliminated the protective effect.
Balb/c mice with estrogen supplementation had a significantly higher rod photoreceptor function in comparison to their control groups (p<0.05). Estrogen treated mice showed 40% and 34% higher rod photoreceptor function at 10db and 6db attenuation in comparison to the vehicle treated group (2A). PHTPP by itself did not lead to further deterioration of rod function (2B); but blunted the protective effects of estrogen when co-administered (2D). Mice treated with estrogen also showed about 20% increase in cone photoreceptor cell function (2C) but co-treatment with PHTPP eliminated the protective effect.
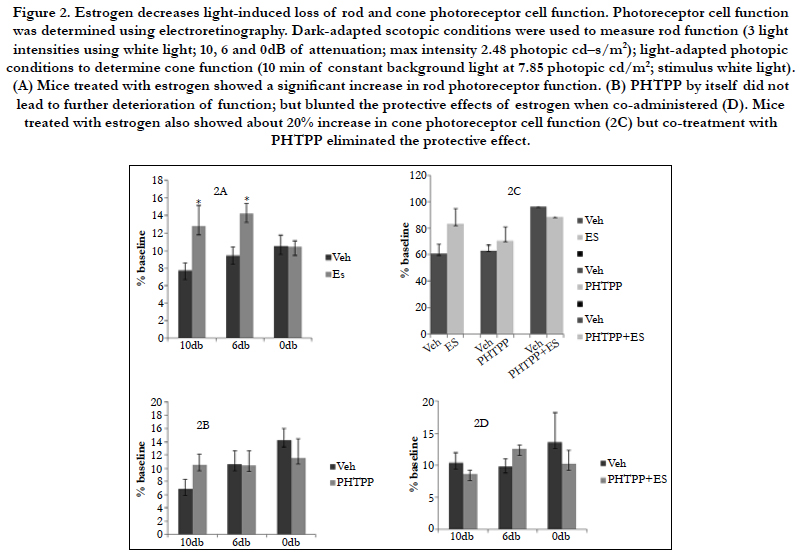
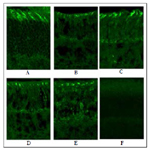
Cone photoreceptor loss is intimately related to vision loss. In mouse, cone function is driven by middle (M, green) and short (S, UV) wavelength cone opsins. Among these two opsins, UV opsin localization and expression is more susceptible to environmental factors [22]. Constant light exposure gradually leads to cone photoreceptor damage. 3 month old Balb/c mice were kept in constant light for 10 days, and one group treated daily with 17-beta estradiol (0.2 mg/kg)(3C), while another group received PHTPP (25μg/kg) every alternate day (3D) and a third group was co-treated with 17-beta estradiol + PHTPP (3E). Control groups were treated with vehicle (3B). Ultra violet (UV) cone opsin immunoreactivity levels were measured in all groups of mice to determine the extent of cone photoreceptor damage. Results indicated lower cone opsin level in the ERβ inhibitor (3D), combination (3E) and vehicle treated mice (3B) whereas estrogen treatment has been found to improve UV opsin immunoreactivity in LD mice (3C). 3 month old Balb/c without LD was used as controls for treatment (3A). UV opsin protein levels were quantified from binarised and thresholded images normalized to a fixed size using Image J software (Figure 3G). Estrogen treatment elevated UV opsin level in light damaged mice. No difference was observed between vehicle control, PHTPP and PHTPP and estrogen co-treated groups.
Figure 3. Estrogen prevents UV cone opsin loss in constant light induced photoreceptor degenerative Balb/c mice.
Discussion
In the present study we have demonstrated that estrogen has a protective role against light induced photoreceptor degeneration. It has been shown previously that Balb/c mice are susceptible to constant light exposure which leads to loss of nuclei in the outer nuclear layer (ONL) and loss of retinal function as assessed by electroretinography. Mice with estrogen supplementation (17β estradiol) lose fewer nuclei in the ONL and exhibited higher rod and cone photoreceptor function. Balb/c mice treated with 17β estradiol also exhibited more UV cone opsin immunoreactivity in the cone outer segments. Functionally, 17β estradiol -treated retinas exhibited better rod photoreceptor function after the 10 day light-damage period. The involvement of estrogen receptor beta (ERβ)-signaling in the protective effects of 17β estradiol treatment were confirmed, documenting that mice co-treated with 17β estradiol and the ERβ-specific antagonist PHTPP no longer exhibited structural and functional improvements.
Continuous light exposure increases oxidative stress [14, 23] and is a major stimulator of retinal diseases [15], particularly dry AMD [24]. The extent of retinal degeneration depends on the light intensity and duration of light exposure [25]. Continuous light also triggers formation and accumulation of toxic photoproducts from vitamin A and leads to photoreceptor cell death [4].
In vivo and in vitro studies have revealed that apart from the estrogen’s role in normal female sex organ development and function [10, 11], estrogen also plays significant role in bone conservation [9], cardiovascular stability [12] and neuroprotection [13, 26]. Estrogen has both anti-oxidative and anti-inflammatory function and it regulates signaling processes in the pathogenesis of AMD. Epidemiological studies have shown that women after menopause are more likely to develop AMD than men, indicating that lower estrogen levels in females after menopause might have an effect on the development of retinal disorders. The purpose of this study is to evaluate the effects of estrogen on photoreceptor cell structure and function in an experimental model for light-induced photoreceptor degeneration.
Estrogen produces diverse biological effects by binding to the estrogen receptor (ER) proteins, further categorized as ER alpha (ERα) and ER beta (ERβ). ERα and ERβ are both nuclear receptors. Both receptors are expressed in the human eye [27, 28]. Recent experimental and clinical data elucidates the importance of the classical estrogen receptors, ERα and ERβ, in the management of retinal disorder pathologies [17, 29].
The molecular mechanism by which estrogen confers its protection against retinal disorders is not yet clear. Boekhoorn and colleagues reported that single nucleotide polymorphisms (SNPs) in ERα are associated with age related macular disorder [30]; whereas for open angle glaucoma, SNPs in ERβ appear to be correlated with disease in female [31]; the Blue Mountains Eye Study reported “that a shorter duration of estrogen production may increase risk of AMD” [32]. In animal studies, reports suggest that estrogen protects RPE cells from oxidative stress mediated injury through ERβ [32]. Our results on constant light-induced photoreceptor cell degeneration in Balb/c showed that the protective effects of estrogen on photoreceptor cell degeneration is mediated in part by the activation of ERβ, a predominant subtype of estrogen receptor in the retina.
Our results showed that the structural and functional impairment of rod and cone photoreceptors after light damage was reduced significantly in mice treated with 17β-estradiol, an effect that eliminated by cotreating the animals with 17β-estradiol and the ERβ inhibitor PHTPP. PHTPP treatment on its own did not augment retinal degeneration. The rod photoreceptor response after light damage was reduced in WT mice by 50% (P<0.001). Our results showed that estrogen treatment significantly (P<0.001) increased the quantity of photoreceptor cell nuclei in ONL compared to the vehicle treated control; contrastingly the ERβ inhibitor treatment reduced (P<0.001) photoreceptor cell numbers in the ONL of retina. Furthermore coadministering PHTPP and 17β-estradiol eliminated the protective effects of estrogen. Rod Photoreceptor cell function was also significantly reduced in light damaged mice (P<0.05), yet estrogen treatment significantly restored rod photoreceptor function. This result also supports the results in the histological studies where PHTPP by itself did not lead to further deterioration of function but blunted the protective effects of 17β-estradiol when co-administered. All these results suggest that the effects of estrogen are mediated by the ER-β receptor on the mice retina. Our results are supported by findings by Wang and colleagues in Sprague-Dawley rats. They demonstrated a protective effect of 17β-estradiol that increases the retinas own anti-oxidant defenses [37]. Kaja S and colleagues also reported a protective role for estrogen in protecting the inner retina from ischemia-induced damage in rat model [33].
Rod photoreceptor survival was evaluated by determining rows of photoreceptor nuclei in the ONL and also by ERG. ERG is a test that measures visual function in response to single light flashes and is performed on each animal before and after light exposure. In Erg, a- wave reflects the outer retinal function. ERG analysis showed good correlation between protection of structure and function; the rod photoreceptor cell response was increased in the estrogen treated group (P<0.05) when compared to the control mice. Rod photoreceptor cell response drives the cone photoreceptor response. The latter was also affected by continuous light exposure and estrogen treatment improved cone cell function. PHTPP alone and in combination showed no alteration as compared to the light damaged mice.
In mice, color vision is mediated by cone photoreceptors, which comprises about 3–5% of cells in the outer nuclear layer [19]. Mice have two types of cone opsins, a short wavelength cone opsin (S opsin) which is also known as UV opsin and a medium to long wavelength (M/L opsin) [34]. Reports suggest that UV or S cones are more vulnerable to aging and diseases [35]. Proper localization and a relative amount of cone opsin protein are required for optimum cone photoreceptor function [20].
Effects of light damage on cone photoreceptor are an indirect effect. Light damage kills rod photoreceptors and impairs cones. UV cone opsin immunoreactivity decreased with light damage as compared to the same aged wild type mice. Our results showed that cones are also responsive to estrogen: UV cone opsin immunoreactivity was found to improve when treated with 17β-estradiol. PHTPP alone and in combination showed no improvement.
Finally, none of the reports to date have compared the effects of estrogen on rods versus cone structure and function. Our results demonstrated a protective effect of 17β estradiol signaling ERβ via on number of photoreceptor nuclei in the ONL as well as on cone opsin expression. The effects on cones might be an indirect effect, reported in other retinal degenerative diseases, in which improved rod survival leads to improved cone survival and function [36]. However, it is plausible that there exist ERβ-mediated intracellular cell signaling pathways in rods and cones.
Conclusion
It was observed that rod and cone photoreceptor structure and function were influenced by the 17β estradiol treatment. Histological results demonstrated a significant difference in the number of rod photoreceptors after estrogen treatment. Additionally, the ERG a- waves were higher in estrogen treated mice as compared to the controls. Patients with dry AMD experience loss of sharp, central vision due to photoreceptor degradation. However, estrogen can protect photoreceptors and thus has therapeutic value in the treatment of AMD and other retinal degenerative disorders.
Acknowledgements
I am thankful to Dr. Bärbel Rohrer for sharing her resources and I am also thankful to Dr. Subhajit Dasgupta for his valuable suggestions on this manuscript.
References
- Algvere PV, Marshall J, Seregard S. Age-related maculopathy and the impact of blue light hazard. Acta Ophthalmol Scand. 2006 Feb;84(1):4-15. Pub- Med PMID: 16445433. doi: 10.1111/j.1600-0420.2005.00627.x.
- Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Opthalmol. 2006 Oct;51(5):461-81. PubMed PMID: 16950247.
- Hao W, Wenzel A, Obin MS, Chen CK, Brill E, Krasnoperova NV, et al. Evidence for two apoptotic pathways in light-induced retinal degeneration. Nat Genet. 2002 Oct;32(2):254-60. PubMed PMID: 12219089.
- Organisciak DT, Vaughan DK. Retinal light damage: mechanisms and protection. Prog Retin Eye Res. 2010 Mar;29(2):113-34. PubMed PMID: 19951742.
- Organisciak, DT, Darrow RM, Barsalou L, Darrow RA, Kutty RK, et al. Light history and age-related changes in retinal light damage. Invest ophthalmol vis sci. 1998 Jun;39(7):1107-1116. PubMed PMID: 9620069.
- Wenzel A, Grimm C, Samardzija M, Reme CE. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog Retin Eye Res. 2005 Mar;24(2):275-306. Pubmed PMID: 15610977.
- Bian M, Du X, Cui J, Wang P, Wang W, et al. (2016) Celastrol protects mouse retinas from bright light-induced degeneration through inhibition of oxidative stress and inflammation. J Neuroinflammation. 2016 Feb 27;13:50. doi: 10.1186/s12974-016-0516-8. PubMed PMID: 26920853.
- Giatti S, Boraso M, Melcangi RC, Viviani B. Neuroactive steroids, their metabolites, and neuroinflammation. J Mol Endocrinol. 2012 Oct 10;49(3):R125-34. doi: 10.1530/JME-12-0127. PubMed PMID: 22966132.
- Cousins SW, Marin-Castano ME, Espinosa-Heidmann DG, Alexandridou A, Striker L, Elliot S. Female gender, estrogen loss, and Sub-RPE deposit formation in aged mice. Invest Opthalmol Vis Sci. 2003 Mar;44(3):1221-9. PubMed PMID: 12601052.
- Elliot S, Catanuto P, Fernandez P, Espinosa-Heidmann D, Karl M, Kenneth k, et al. Subtype specific estrogen receptor action protects against changes in MMP-2 activation in mouse retinal pigmented epithelial cells. Exp Eye Res. 2008 Apr;86(4):653-60. doi: 10.1016/j.exer.2008.01.010. PubMed Central. PMCID: PMC2409190.
- Yu X, Tang F, Li F, Frank MB, Huang H, Zhu Y, et al. Protection against hydrogen peroxide-induced cell death in cultured human retinal pigment epithelial cells by 17beta-estradiol: a differential gene expression profile. Mech Ageing Dev. 2005 Nov;126(11):1135-45. PubMed PMID: 16029884.
- Espinosa-Heidmann DG, Marin-Castano ME, Pereira-Simon S, Hernandez EP, Elliot S, Cousins SW. Gender and estrogen supplementation increases severity of experimental choroidal neovascularization. Exp Eye Res. 2005 Mar;80(3):413-23. PubMed PMID:15721623.
- Feskanich D, Cho E, Schaumberg DA, Colditz GA, Hankinson SE. Menopausal and reproductive factors and risk of age-related macular degeneration. Arch Opthalmol. 2008 Apr;126(4):519-24. doi: 10.1001/archopht. 126.4.519. PubMed PMID: 18413522.
- Blanks JC, Pickford MS, Organisciak DT. Ascorbate treatment prevents accumulation of phagosomes in RPE in light damage. Invest Opthalmol Vis Sci. 1992 Sep;33(10):2814-21. PubMed PMID: 1526731.
- Mitra RN, Conley SM, Naash MI. Therapeutic approach of nanotechnology for oxidative stress induced ocular neurodegenerative diseases. Adv Exp Med Biol. 2016;854:463-9. doi: 10.1007/978-3-319-17121-0_62. PubMed PMID: 26427447.
- Dimitrova KR, DeGroot KW, Suyderhoud JP, Pirovic EA, Munro TJ, Kim YD, et al. 17-beta estradiol preserves endothelial cell viability in an in vitro model of homocysteine-induced oxidative stress. J Cardiovasc Pharmacol. 39(3):347-53. 2002 Mar;39(3):347-353. PubMed PMID: 11862113.
- Kobayashi K, Kobayashi H, Ueda M, Honda Y. Estrogen receptor ezpression in bovine and rat retinas. Invest Opthalmol Vis Sci. 1998 Oct;39(11):2105- 10. PubMed PMID: 9761289.PubMed PMID: 9761289.
- Lohr HR, Kuntchithapautham K, Sharma AK, Rohrer B. Multiple, parallel cellular suicide mechanisms participate in photoreceptor cell death. Exp Eye Res. 2006 Aug;83(2):380-9. PubMed PMID: 16626700.
- Richards A, Emondi AA, Rohrer B. Long-term ERG analysis in the partially light-damaged mouse retina reveals regressive and compensatory changes. Vis Neurosci. 2006 Feb;23(1):91-7. PubMed PMID: 16597353.
- Bandyopadhyay M, Kono M, Rohrer B. Explant cultures of Rpe65-/- mouse retina: a model to investigate cone opsin trafficking. Mol Vis. 2013 May;19:1149-57. PubMed PMID: 23734084.
- Bandyopadhyay M, Rohrer B. Photoreceptor structure and function is maintained in organotypic cultures of mouse retinas. Mol Vis. 2010;16:1178-85. PubMed Central PMCID: PMC2901185.
- Rohrer B, Lohr HR, Humphries P, Redmond TM, Seeliger MW, Crouch RK. Cone opsin mislocalization in Rpe65-/- mice: a defect that can be corrected by 11-cis retinal. Invest Opthalmol Vis Sci. 2005 Oct;46(10):3876-82. PubMed PMID: 16186377.
- Taguchi H, Ogura Y, Takanashi T, Hashizoe M, Honda Y. In vivo quantitation of peroxides in the vitreous humor by fluorophotometry. Invest Opthalmol Vis Sci. 1996 Jun;37(7):1444-50.
- Hnaus J, Zhao F, Wang S. Current therapeutic developments in atrophic agerelated macular degeneration. Br J Opthalmol. 2016 Jan;100(1): 122-7. doi: 10.1136/bjophthalmol-2015-306972corr1. PubMed PMID: 26553922.
- Chen JQ, Cammarata PR, Baines CP, Yager JD. Regulation of mitochondrial respiratory chain biogenesis by estrogens/estrogen receptors and physiological, pathological and pharmacological implications. Biochim Biophys Acta. 2009 Oct;1793(10):1540-70. doi: 10.1016/j.bbamcr.2009.06.001. PubMed PMID: 19559056.
- Jeon CJ, Strettoi E, Masland RH. The major cell populations of the mouse retina. J Neurosci. 1998 Nov 1;18(21):8936-46. PubMed PMID: 9786999.
- Munaut C, Lambert V, Noel A, Frankenne F, Deprez M, Foidart J, et al. Presence of oestrogen receptor type beta in human retina. Br J Opthalmol. 2001 Jul;85(7):877-82. PubMed Central PMCID: PMC1724050.
- Ogueta SB, Schwartz SD, Tamashita CK, Farber DB. Estrogen receptor in the human eye: influence of gender and age on gene expression. Invest Opthalmol Vis Sci. 1999 Aug;40(9):1906-11. PubMed PMID: 10440242.
- Kaarniranta K, Machalinska A, Vereb Z, Salminen A, Petrovski G, Kauppinen, A. Estrogen signalling in the pathogenesis of age-related macular degeneration. Curr Eye Res. 2015 Feb;40(2):226-33. PubMed PMID: 24911983. doi: 10.3109/02713683.2014.925933.
- Boekhoorn SS, Vingerling JR, Uitterlinden AG, Van Meurs JB, van Duijn CM, Pols HA, et al. Estrogen receptor alpha gene polymorphisms associated with incident aging macula disorder. Invest Opthalmol Vis Sci. 2007 Mar;48(3):1012-7. PubMed PMID: 17325140.
- Mabuchi F, Sakurada Y, Kashiwagi K, Yamagata Z, Iijima H, Tsukahara S. Estrogen receptor beta gene polymorphism and intraocular pressure elevation in female patients with primary open-angle glaucoma. Am J Opthalmol. 2010 May;149(5):826-30. PubMed PMID: 20399928. doi: 10.1016/j.ajo.2009.12.030.
- Giddabasappa A, Bauler M, Yepuru M, Chaum E, Dalton JT, Eswaraka J. 17-beta estradiol protects ARPE-19 cells from oxidative stress through estrogen receptor-beta. Invest Opthalmol Vis Sci. 2010 Oct;51(10):5278-87. PubMed PMID: 20463317. doi: 10.1167/iovs.10-5316.
- Kaja S, Yang SH, Wei K, Fujitani K, Liu R, Brun-Zinkernagel AM, et al. Estrogen protects the inner retina from apoptosis and ischemia-induced loss of Vesl-1L/Homer 1c immunoreactive synaptic connections. Invest Opthalmol Vis Sci. 2003 Jul;44(7):3155-62.
- Cunea A, Powner MB, Jeffery G. Death by color: differential cone loss in the aging mouse retina. Neurobiol Aging. 2014 Nov;35(11):2584-91. PubMed PMID: 24929970. doi: 10.1016/j.neurobiolaging.2014.05.012.
- Cho NC, Poulsen GL, Ver Hoeve JN, Nork TM. Selective loss of S-cones in diabetic retinopathy. Arch Opthalmol. 2000 Oct;118(10):1393-400. Pub- Med PMID: 11030822.
- Piano I, Novelli E, Gasco P, Ghidoni R, Strettoi E, Garginic C. Cone survival and preservation of visual acuity in an animal model of retinal degeneration. Eur J Neurosci. 37(11):1853-62. PubMed PMID: 23551187. doi: 10.1111/ejn.12196.
- Wang S, Wang B, Feng Y, Mo M, Du F, et al. 17 beta estradiol ameliorates light-induced retinal damage in Sprague-Dawley rats by reducing oxidative stress. J Mol Neurosci. 55(1):141-151. doi: 10.1007/s12031-014-0384-6.