Intraoperative Injection of Triamcinolone Acetonide in External Dacryocystorhinostomy
Vasu N Naik1*, Vijay Kumar2
1 Ophthalmology Department, District Hospital, NHM, District Health and Family Welfare Office, Chamarajanagara District, Karnataka, India.
2 Malleswaram Eye Hospital, 1 Floor, Radha Building, Sampige Road Malleswaram Circle, Banglore, Karnataka, India.
*Corresponding Author
Vasu N Naik,
Ophthalmology Department, District Hospital, NHM, District Health and Family Welfare Office, Chamarajanagara District, Karnataka, India.
Tel: 9449853973
E-mail: gunjanna@gmail.com
Received: July 30, 2020; Accepted: August 18, 2020; Published: August 21, 2020
Citation: Vasu N Naik, Vijay Kumar. Intraoperative Injection of Triamcinolone Acetonide in External Dacryocystorhinostomy. Int J Ophthalmol Eye Res. 2020;8(2):424-428. doi: dx.doi.org/10.19070/2332-290X-2000086
Copyright: Vasu N Naik© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Aim: A prospective and interventional study to known the efficacy and complications of intraoperative triamcinolone acetonide
(TMA) 40mg/ml injection in primary external dacrycystorhinostomy ( EXDCR).
Material and Methods: Clinically confirmed 38 cases of primary acquired nasolacrimal duct obstruction (PANDO) are
randomly grouped as A (Study group) with 20 cases and B (Control group) with 18 cases. Both group underwent primary
EXDCR with silicone tube(ST) intubation. Group A cases received 0.5 ml of TMA 40mg/ml and group B 0.5 ml of Gentamycin
40mg/ml injection intraoperatively. Resolution of symptoms and complications of TMA injection are evaluated at 6
months follow up.
Result: Mean age, gender and laterality of the eye involvement are not different between two group. The success rate in
group A is 90%(18/20) and group B 88.9% ( 16/18), the difference is very small (P=1.0). Raised IOP (Intraocular pressure )
of > 18mmHg observed in 10% (2/20 ) cases of group A. Group A cases had minimal post operative wound inflammation
80%(16/20 ) compare to 44% (8/18) cases in group B.(P= 0.04). Early resolution with cosmetically good scar seen in 90%
(18/20) in group A compare to 61% ( 10/18) cases in group B (P=0.02).
Discussion: Intraoperative TMA injection does not improve the success rate of primary EXDCR in PANDO. But significantly
reduces the immediate postoperative wound inflammation and facilitates early resolution of the wound scar. Raised
IOP is a concern in TMA injection group.
2.Introduction
3.Material and Method
4.Results
5.Discussion
6.References
Keywords
Benefits; Complications; EXDCR; Triamcinolone.
Introduction
Epiphora, discharge and painless swelling in the medial canthal
area are the common presentations of primary acquired nasolacrimal
duct obstruction (PANDO) [1, 2]. The gold standard treatment
for PANDO is external dacryocystorhinostomy (EXDCR )
with success rate of 59% - 99% [3-6].
Various factors have been blamed for the failure of EXDCR.
Absence of adequate size flaps in anastomosis, reduced size of
osteotomy opening, fibrotic closing of the common canalicular
opening, intra nasal synechia, narrowing or the closure of the osteal
opening, abnormal position of the lacrimal sac wall and nasal
mucosal anastomosis, granuloma formation at the inner opening
of the anastomosis, exposed bony margin facilitating granulation
tissue formation and racial people with higher melanin in the
body [2, 5, 7-11].
Many modalities and modifications have been studied to overcome
these predisposing factors. Altering the number of dacrycystorhinostomy
(DCR) flaps and increasing the size of osteotomy
[8, 12], intraoperative use of Mansoura T tube [13], silicone
tube intubation [3, 4, 14], use of antifibrotic agents like Mitomycin
C (MMC), 5-Flurouracil and depot steroids [9-11, 15].
Depot steroid, Triamcinolone acetonide (TMA) has been widely
used in many ophthalmic diseases and procedures. As intralesional
injection in medical management of chalazion [16, 17], as retobulbar
injection in management of non responding dysthyroid
proptosis [18], in management of diabetic macular oedema [19],
for visualizing Vitreous during vitrectomy [20], and in treatment periocular scars [21]. Considering its anti inflammatory and antifbrotic
action a hypothesis was made that intra operative depot
steroid TMA may reduce the post operative inflammation related
complications and improve the success rate of EXDCR in PANDO.
Material and Method
This is a prospective, comparative and interventional study done
in the District Hospital Chamarajanagara in Karnataka state. This
study was carried from August 2017 to July 2019. The study was
approved by the review and ethical committee of the hospital.
All patients were informed about the merits and demerits of the
study and written consent was taken. The study adhered to declaration
of Helsinki 1975. Two group were made, Group A (study
group) undergoing EXDCR + silicone tube intubation with intraoperative
0.50 ml of undiluted TMA 40mg/ml depot steroid injection
(Aurocort, Aurolab, Madurai, India) and Group B (control
group) undergoing EXCDR + silicone tube intubation with intraoperative
0.50 ml of Gentamycin 40mg/ml injection. (Gentalab,
Laborate Pharmaceutical India Ltd. Patna Sahib, India).
All enrolled patients’ demography and detailed history was recorded.
All patients under went comprehensive eye examination.
Lacrimal duct system related diagnostic probe test, syringing, Fluorescein
Dye Disappearance(FDD) test are done. For tear function
Schirmer test and Break Up Time( BUT) were also done.
Based on probe test, syringing and FDD test PANDO was confirmed.
Patients were randomly allotted into study group and control
group. Exclusion criteria are acquired secondary nasolacrimal
duct block, punctual stenosis, trichiasis, entropion, ectropion, lag
ophthalmos, chronic blapheritis, dry eye, corneal surface disorder.
Patients with comorbid systemic diseases, nasal pathology and age
group below 18 years were excluded from study.
All Patients were operated from single surgeon .Surgical steps
of EXDCR are as described by Dupuy - Dutemps and Bourget
modified with only single anterior flap in lacrimal sac and nasal
mucosa [8, 12]. Under sterile condition surgical area infiltrated
with 2:1 ratio mixtured of 2% lidocaine and 0.5% bupivacaine.
Nasal pack done with 1.25cm size ribbon gauze soaked in 30 ml
4% xylocaine with 2 ampule of adrenaline 1:10000 and left till the
time of silicone stenting. A curved 10-12 mm size incision 8-10
mm from medial canthus taken all along the direction of anterior
lacrimal crest starting from middle point of the medial palpebral
ligament. Layer by layer skin, subcutaneous tissue, orbicularis
oculi were separated to expose the medial end medial palpebral
ligament. Medial palpebral ligament disinserted. Lamina papyracea
was gently perforated with bone rongue. Around 10 -15 mm
diameter size osteotomy done with serial sized bone rounge. With
the Bowmans probe 00 medial wall of lacrimal sac tented and
single large anterior flap was made .Same size anterior flap made
in nasal mucousa.A bicanalicular Silicone tube (Aurolac-from Aurolab,
Madurai, India) intubated through upper and lower pucta,
retrieved through common canaliculi, osteotomy and into nasal
cavity. Two ends of the tube were tied in such way that there is
no snaring effect on punctae, unnecessary looping into medial
fornix or hanging out of the nostril. Tied end of the tube left
free in the nasal cavity at the inferior turbinate level. Both the flap
were trimmed as per the requirement and joined together with 6 0
vicryl. At this point undiluted 0.50 ml of TMA 40mg/ml injection in group A and 0.50 ml of Gentamycin(GM) 40mg/ml injection
in group B was injected. Injection was given all around the bony
opening between nasal mucosa and bone endostium and 0.1 to
0.2 ml to fill the empty space above the anastomosis. Palpebral
ligament refixed, orbicularis muscle and skin were closed. For all
suturing 6- 0 vicryl used . Fresh nasal pack and pad bandage applied.
Post operatively all patients received oral Dcilofenac +paracetamol,
cefixime 200 mg twice daily for 5 days . Topically antibiotic
drops five times daily in group A and antibiotic steroid drops five
times daily in group B. Antibiotic ointment application over the
wound two times daily in both group. Follow up done on 1 day,1
week, 4 week and monthly for 6 months . Nasal pack was removed
on first post operative day, skin sutures on 10th day and
silicone tube was removed between 12 – 24 weeks post operatively.
During each visit patients were evaluated for the success
and complications of the procedure. Success of the procedure
was considered when both subjective and objective resolution of
PANDO. Subjectively resolution of epiphora or discharge. Objectively
by anatomical and functional patency of anastomosis by
syringing and FDD test respectively.
The results are analyzed by NCSS 2020 Statistical Software (2020).
NCSS, LLC. Kaysville, Utah, USA and The results are presented
with group mean compared with two tailed Fishers exact test and
statically significance by actual P value.
Results
There were 38 eyes from 38 patients . Mean age of the patients
is 48.8+/-.15.4 (20 – 72) yrs. Female male ratio is 1.5:1. Right eye
was involved in 47.4%( 18/38) patients and left eye in 52.6% (
20/38) patients and difference is very narrow ( P=0.35) . Common
presentation was epiphora in 73.6% ( 28/38) patients, mucopurulent
discharge in 18.4% (7/38) and mucocele in 8% (3 /38)
patients .Mean duration of presentation is 9.2 (13 – 38 ) months.
Around 89.5% (34/38) patients were having both subjective and
objective resolution of symptoms (epiphora/discharge/mucocele)
at the end of 6 months. Only 10.5% (2/38) patients had
no resolution of the symptoms both subjectively and objectively.
In 5.3% (2/38) patients IOP was raised > 15 mmHg from base
line reading during the follow up period.
There were 20 patients in group A and 18 patients in group B.
Mean age of group A patients was 46.5+/- 15.3 and appears to
be younger than mean age of group B patients 51.6+/-15.6 yrs,
but the difference is very small and not relevant (P=0.35) There
is no much difference in the gender affection female : male ratio
was 1.5:1 and 1.6:1( P=1.00), laterality of the affected eye R/E :
L/E 11:9 and 7:11 (P=0.35) respectively between group A and
B. There is no difference in the Presenting symptoms and duration
of symptoms between two groups. (P=0.71). (Table 1). On
comparing the success rate between two group, in group A 90%
(18/20) cases and in group B 88.9% (16/18) cases were having
subjective and objective resolution of symptoms at the end 6
months and the difference is to small (P=1.00).
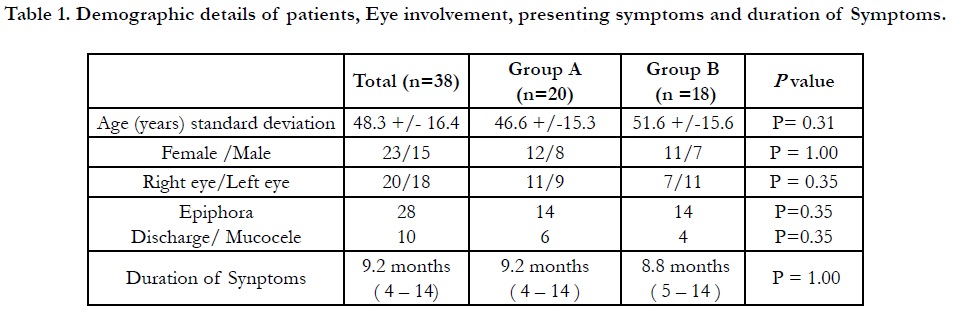
Table 1. Demographic details of patients, Eye involvement, presenting symptoms and duration of Symptoms.
There were 10% (2/20) cases of failure in group A. Both after the removal of the stent at 4th month follow up and underwent re-intubation of the stent. In group B the procedure was failed in 11.1% (2/18) cases. These two cases had adhesion in the nasal cavity with the stent in situ on 3rd month follow up . Both the cases received 0.50 ml of TMA 40mg/ml injection and both cases had resolution of symptoms. All These 4 cases were treated as failed cases.
In group A 10% (2/20) cases had raised IOP. One case on 2nd month follow up period. Applanation IOP was >15 mmHg more than base line IOP and controlled with topical Beta blocker, after 6 weeks treatment IOP stabilized to <21 mmHg. Another patient on 3rd month follow up. The raise was > 22 mm of Hg from base line IOP and controlled with Beta blocker and alpha agonist to < 21 mm Hg and patient converted to steroid induced glaucoma.
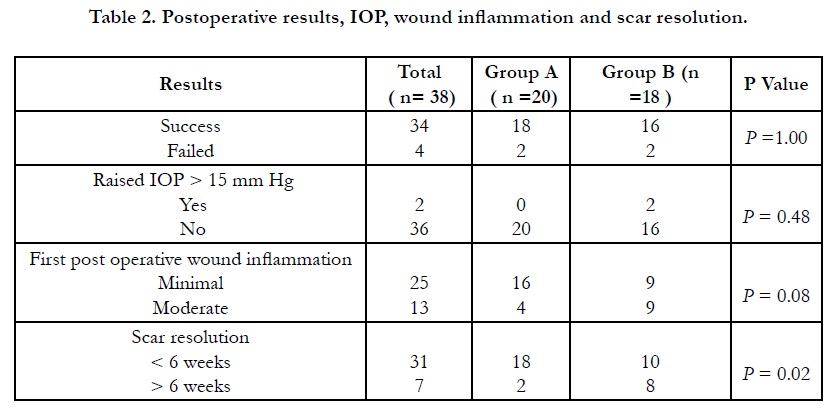
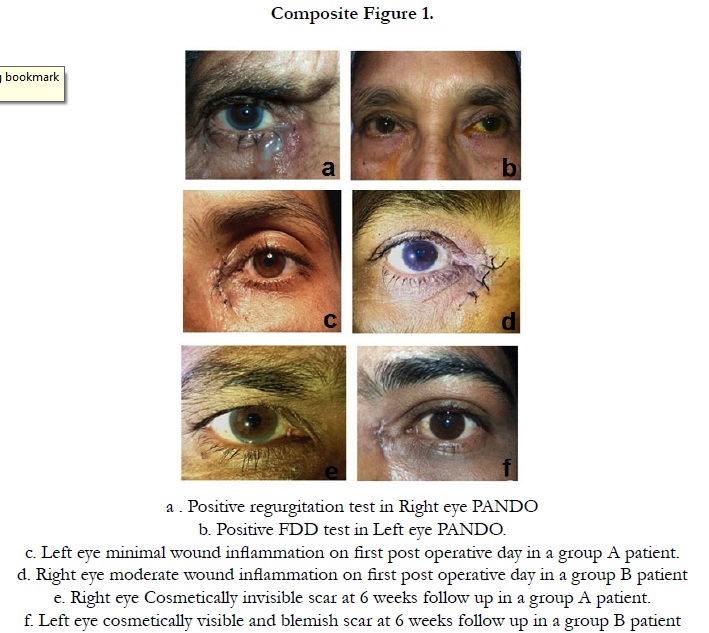
Other observations we have observed in this study was minimal post operative inflammation around the incision wound in 80% (16/20) cases in group A compare to group B 44% (8/18) with wide difference between two groups (P=0.04). It is to be remembered that we have not used topical steroid drop in group A patients for 6 weeks and the local anti-inflammatory effect was taken care by depot steroid. Regarding the wound related scar it was cosmetically invisible in 90% (18/20) cases in group A and 56% (10/18) in group B and the difference is large. (P=0.02).(Table .2) (Composite Figure .1)
Discussion
Depot steroid, TMA is a synthetic corticosteroid having 5 times
anti-inflammatory action compare to hydrocortisone. It is a long
acting anti-inflammatory, Anti Vascular endothelial growth factor
(VEGF) and antifibrotic agent. Mode of action is on chemical
mediators by inhibiting phospholipase A2 which in turn prevents
the synthesis of thormbaxane A, prostaglandins and leukotrenes.
Also it is a inhibitor of inflammatory cells, inhibitor of VEGF
gene and reduces the capillary permeability. These functions reduces
exudation of plasma fluids, controls the fibroblast proliferation
and regulates collagen deposit [21, 22]. An assumption was
made that intraoperative depot steroid may reduces the inflammation,
prevents granulation tissue formation, minimizes the fibrosis
and scar formation after EXDCR in cases of PANDO.
The mean age of the patients was 48.8 (20-72) yrs. This study had
more younger patients compare to previous report of mean age
of 56 yrs [8, 13] and the difeerence is small (P=0.25) Majority of
our patients were from rural area and farmers . Constant exposure
to irritants like sun light, pollens, animal hairs and dust particles
are known to alter the chemical composition of tear and nasal secretion. These altered composition cause anatomical changes in
mucosal lining of the lacrimal drainage system and nasal mucosal
epithelium. These factors indirectly affect the cavernous plexus
as follows: descending inflammation from the eye or ascending
inflammation from the region of the nose may initiate malfunctions
in the cavernous body with reactive hyperemia, swelling of
the mucous membrane, and temporary occlusion of the lacrimal
passage. Then, repeated isolated episodes of dacryocystitis may
lead to structural epithelial and subepithelial changes. Loss of
typical goblet and epithelial cells, which plays an important role in
the tear-outflow mechanism, as well as fibrosis of the helical system
of connective tissue fibers in the area of the lacrimal sac and
nasolacrimal duct. Addition to this there is reduction and destruction
of specialized blood vessels of the cavernous body leading
to malfunctions of the tear-outflow mechanism and the vicious
circle continues [1, 2]. This may be the reason for more number
of younger patients developing PANDO in our study. On gender
involvement female - male ratio was 1.5:1 and this is comparable
to earlier observation of PANDO is more common in females
[3, 4]. This study did not find any difference in the right eye and
Left eye involvement of PANDO 47.36% (18/38 ) and 52.64%
(20/38) respectively (P=0.35).
Epiphora was the common symptoms in our study 73.68%
(28/38 cases) this is in concordance with earlier reports [3, 4, 13].
The observed success rate of 89.47% ( 34/38 ) in our study is
comparable with previous reports [4, 5, 13].
The mean age, gender involvement, laterality of the eye involvement
and duration of symptoms are not different between two
group (Table.1).
With the available literature common factors for failed EXDCR
are fibrotic block of the common canalicular opening, adhesion
in the nasal cavity and closure of the osteal opening by granulation
tissue [1, 2]. Depot steroid TMA is known to reduce the inflammation, granulation tissue formation and fibrotic tissue [9].
The assumption in the beginning of this study was the depot steroid
should improve the success rate of EXDCR in TMA received
group compare to GM injection received group.
The success rate in study group was 90 % (18/20)compare to
control group 89% ( 16/18) with small difference (P=1.0). This
success rate of 90% (18/20) is comparable to previous study report
[11]. Aerin et al., [11] in their prospective study, 45 patients
with PANDO underwent endonasal DCR. During the follow up
period various type of granuloma developed at various location
of the ostium. They were treated with intralesional injection of 0.3ml of TMA 40mg/ml and the final success rate was 75.55 %
(34/45 cases). In their study steroid was injected during the follow
up period and in endonasal DCR osteotomy size relatively
small and mucosal flaps are resected without anastomosis .These
factors may be the reason for more number of failed cases in
their study. It has been observed that depot steroid is more beneficial
during the initial period of inflammation in reducing the
fibrosis, scar formation and granulation tissue development [22].
Another explanation may be that we have used silicone stent in
both the group. The stent it self has many positive out come on
EXDCR. It enhances the lacrimal pump action, well opposes the
puncta which improves the lacrimal flow during the closing phase
of blinking, increased capillary function of canaliculi, prevents
granuloma formation, by delaying the fibrosis, reduces the scarring
and adhesion around the osteal opening [5, 12, 14]. However
this difference is minimal (P=0.31).
Where as in a retrospective study Lee et al., [15] 15 eyes of PANDO
5 cases received introperative TMA and 100% (5/5) had
complete resolution of PANDO . Even thou Lee et al., [15]. reported
success rate is more than our success rate of 90% (18/20)
the difference is not significant (P=1.0). Moreover Lee et al., [15]
patients were on systemic immunosuppressive agents for sarcoidosis,
this may be reason for higher success rate.
Two patients in study group had raised IOP of more than 15 mm
Hg during the follow up period. In general population 5-6 % will
have raised IOP of more than 15 mm Hg for local and periocular
steroid preparation and this will be 29 -30 % in primary open
angle glaucoma patients [23]. We suggest that comprehensive glaucoma evaluation to be done before using the depot steroid in
EXDCR patients.
We have noticed the following benefits in depot steroid received
group. There was no requirement of topical steroid drops, post
operative minimal inflammation around the incision wound and
early resolution of wound scar. In a prospective study by Tanushree
et al., [21] Nine patients had post DCR hypertropic scar and
each received 0.2-0.4 ml of TMA 40mg/ml 2-3 injections into
the scar at 6 weekly interval. All the patients had resolution of
hypertrophic scar. In our study 0.3-0.4 ml depot steroid was injected
around osteotomy and 0.1-0.2 ml was pooled over the flap
anastomoses before skin - muscle layers closed. This might have
had positive outcome on scar development.
Even thou our study has failed to prove the assumption of intra
operative injection of TMA will improve the positive outcome of
EXDCR (P=1.00). It has shown other non studied benefits like
reduced postoperative inflammation, omission of topical steroid
and early resolution of scar. Only concern is raised IOP in
susceptible patients and converting to steroid induced glaucoma.
Comprehensive glaucoma eye examination to be a manadatory in
patients receiving TMA EXDCR procedure. Lacunae in our study
is small sample size.
References
- Paulsen F. Pathophysiological aspects of PANDO, dacryolithiasis, dry eye, and punctum plugs. InAtlas of lacrimal surgery. 2007; 15-27.
- Weber RK, Keerl R, Schaefer SD, Rocca RC. Atlas of lacrimal surgery. Springer Science & Business Media. 2007 Jan 19; chapter 3: 29-52.
- Monka A, Zhungli S. Silicone Intubation in External Dacryocystorhinostomy. International J of Science and Research. 2015 Dec; 4(12): 1814-6.
- Saiju R, Morse LJ, Weinberg D, M K Shrestha, S Ruit. Prospective randomized comparison of external DCR with an without silicone tube intubation. Br J Ophthalmol. 2009; 93:1220 -1222. PMID: 19515642.
- Nuhoglu F, Sarıcı K, Ozdemir F E. Outcome of External Dacryocystorhinostomy with Bicanalicular Silicone Tube Stenting. JAREM. 2014; 4(3).
- Anatolia AM. The effect of silicone tube intubation in external Dacryocystorhinostomy. Acta Med Anatol. 2015; 3(1):1-4.
- Neena MP, Andrew RP. External dacryocystorhinostomy for the treatment of epiphora in patients with patent but non-functioning lacrimal systems. Br J Ophthalmol 2010; 94: 233-35. PMID: 19692388.
- Bothra N, Wani RM, Ganguly A, Tripathy D, Rath S. Primary nonendoscopic endonasal versus external dacryocystorhinostomy in nasolacrimal duct obstruction in children. Indian J Ophthalmol 2017; 65: 1004-7. PMID: 29044069.
- Mukhtar S A, Jamil A Z, Ali Z. Efficacy of External Dacryocystorhinostomy (DCR) with and without Mitomycin-C in Chronic Dacryocystitis. Journal of the College of Physicians and Surgeons Pakistan. 2014; 24(10): 732-735. PMID: 25327916.
- Nair AG, Ali MJ. Mitomycin C in Dacrycystorhinostomy: From experimental to implementation and the road ahead: A Review. Ind J ophthalmol. 2015; 63: 335-9. PMID: 26044474.
- Aerin Jo A, Lee S H, Song W C, Shin H J. Effects of ostium granulomas and intralesional steroid injections on the surgical outcome in endoscopic dacryocystorhinostomy. Graefe's Archive for Clinical and Experimental Ophthalmology. 2018; 256(10): 1993-2000. PMID: 29858678.
- Pradeep B, Rajendran B. Double canalicular single tube intubation in DCR. In J Ophthalmol 1983; 31: 329 -30.
- Elhesy A. Effect of lacrimal silicone intubation in persistant epiphora after an anatomically successful but functionally failed dacryocystorhinostomy (Mansoura technique). Journal of Egyptian Ophthalmological Society. 2013; 106: 74–77.
- Connell P P, Fulcher TP, Chacko E, Connor M J O, Moriarty P. Long term follow up of nasolacrimal intubation in adults. Br J Ophthalmol. 2006; 90: 435–436. PMID: 16547322.
- Lee BJ, Nelson CC, Lewis CD, Perry JD. External dacryocystorhinostomy outcomes in sarcoidosis patients. Ophthal Plast Reconstr Surg. 2012; 28: 47–49. PMID: 22082600.
- Sloas HS, Starling J, Galentine PG, Hargett NA. Treatment of chalazion with injectable triamcinolone. Ann Ophthalmol. 1983; 15: 78- 80.
- Ho SY, Lai JSM. Subcutaneous steroid injection as treatment for chalazion: Prospective case series. KKMJ. 2002; 8: 18 – 20. PMID: 11861988.
- Hadas Stiebel-Kalish, Eyal Robenshtok, Murat Hasanreisoglu, David Ezrachi, Ilan Shimon, Leonard Leibovici. Treatment modalities for Grave’s ophthalmopathy: systemic review and metaanalysis. J Clin Endocrinol Metab. 2009; 94: 2708-2716. PMID: 19491222.
- Ockrim ZK, Sivaprasad S, Falk S, S Roghani, C Bunce, Z Grego, et al. Intravitreal Triamcinolone versus laser photocoagulation for persistant diabetic macular oedema. Br J Opthalmol. 2008; 92: 795-799. PMID: 18420749.
- Burk SE, Da Mata AP, Snyder ME, Susan Schneider, Robert H Osher, Robert J Cionni. Visualising vitreous using Kenalog suspension. J cataract Refract Surg. 2003; 29: 645-651. PMID: 12686230.
- Tanushree V, Venkategouda HT. 5 Fluorouracil versus Triamcinolone injecion in the management of Periocular scar. IJOER. 2016; 4(6): 231- 232.
- Bartlett JD, Jaanus SD. Clinical ocular pharmacology. Elsevier Health Sciences; 2007 Nov 12.
- Redmond J H Smith. Chandler and Grant’s Glaucoma. Br J Ophthalmol. 1987 Jan; 71(1): 76.