Systemic Fat Embolism-Induced Accumulation of Fat Droplets in the Rat Retina
Caroline G. Olson1, Landon J. Rohowetz1, A. Paula Monaghan-Nichols2, Alan Poisner3, Agostino Molteni4, Peter Koulen1,2,5*
1 Vision Research Center, University of Missouri – Kansas City, School of Medicine, Kansas City, 7 Missouri, USA.
2 Department of Biomedical Sciences, University of Missouri – Kansas City, School of Medicine, Kansas City, Missouri, USA.
3 University of Kansas Medical Center, Kansas City, Kansas, USA.
4 Department of Pathology, University of Missouri- Kansas City, School of Medicine, Kansas City, Missouri, USA.
5 Vision Research Center, University of Missouri – Kansas City School of Medicine, Department of Ophthalmology, Kansas City, Missouri USA.
*Corresponding Author
Peter Koulen,
Vision Research Center, University of Missouri – Kansas City School of Medicine, Department of Ophthalmology, Kansas City, Missouri USA.
Tel: 816-235-6733
E-mail: koulenp@umkc.edu
Received: June 09, 2022; Accepted: July 14, 2022; Published: July 27, 2022
Citation: Caroline G. Olson, Landon J. Rohowetz, A. Paula Monaghan-Nichols, Alan Poisner, Agostino Molteni, Peter Koulen. Systemic Fat Embolism-Induced Accumulation of Fat Droplets in the Rat Retina. Int J Ophthalmol Eye Res. 2022;10(3):484-488.
Copyright: Peter Koulen© 2022. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Fat embolism syndrome has been implicated in damage to the blood-retina barrier and retinopathies, thereby representing a potential target for therapy development. In order to characterize features of retinal emboli including size, number, and location, an established rat model of pulmonary fat embolism was employed. Adult rats received caudal vein injections of triolein and the short term (2 days) and long term (70 days) consequences of systemic fat emboli were examined histologically in the retina. Significant numbers and distribution of emboli were observed in the retina at 2 days but the percent and location of emboli in the retina from experimental animals at 70 days was not significantly different from controls. We conclude that systemic fat embolism can cause fat droplet emboli in the retina for brief periods of time, but that they resolve with time.
2.Introduction
3.Materials and Methods
4.Results
5.Discussion
6.Acknowledgments
7.References
Introduction
Triolein; Fat Embolism Syndrome; Retina; Emboli; Traumatic Injury.
Introduction
Systemic fat embolism induced by intravenous injection of triolein
results in the accumulation of fat droplets in pulmonary
tissue leading to septal and arterial inflammation and eventually
pulmonary fibrosis in rats.[1] A cat model of fat emboli led to a
significant disruption of the blood-retina barrier.[2] Clinical observations
indicate a potential involvement of fat embolisms in
retinopathy [3-7], presenting a potential target for therapy development.[
8] This is of particular significance because retinopathy
is a leading cause of irreversible blindness in people over 50 years
of age in the developed world.[9, 10] Retinopathies are separated
into non-neovascular (dry, atrophic, or nonexudative) and
neovascular (wet, or exudative) forms. While neovascular retinopathy
represents only about 10-15% of cases, it constitutes over
80% of severe vision loss associated with retina degeneration.
[9, 10] Numerous causes of pathogenesis have been postulated
including ischemia.[10] Several studies have linked embolisms
and micro-embolisms and subsequent cellular signaling pathways
with ischemic disease.[2-7, 9, 10] In patients with fat embolism
syndrome (FES), fifty percent experienced neovascular retinopathy.
Retinopathy seen in FES resembles diabetic retinopathy
and includes macular edema, cotton wool spots, and bilateral 48
intraretinal hemorrhages.[11, 12] While fat emboli are known to
cause relatively less damage than other types of emboli, the inflammatory
effect along with the mechanical damage and local
ischemia can cause lasting damage.[2] This ischemia can trigger
release of VEGF leading to activation of an angiogenic cascade
with neovascularization and subsequent intraretinal hemorrhages.
These hemorrhages can obscure vision and cause macular edema.
The most common lasting visual deficits are paracentral visual
scotomas which can negatively impact patient’s activities of daily
living and quality of life.[12] This study utilizes a rat model of fat
embolism syndrome utilizing triolein injection at two time points
time from injection to sacrifice to examine the characteristics of
the emboli including size, number, and location of emboli in the
retina. Acute (2 days) and long term (70 days) consequences of
triolein injection were examined in order to better understand the
timeline of the disease.
Material and Methods
This study was conducted under the approval of the University of
Missouri at Kansas City Institutional Animal Care and Use Committee
(IACUC). Animal care and procedures were in accordance
with institutional guidelines. All rats were given ad libitum access
to food and water, and observed at various time points after the
injections. No animals died before euthanasia using inhalation isoflurane
(Sigma Corp., St. Louis, MO).
The histology material was prepared from a total of 19 male
Sprague- Dawley rats (Harlan Laboratories, Indianapolis, IN;
body weight, approximately 300g) divided into 2 cohorts: Rats received
an intravenous tail vein injection of either 0.2ml of sterile
physiological saline or 0.2ml pure triolein (glyceryl trioleate, Sigma
grade; Sigma Corp., St. Louis, MO) and were euthanized 2 days
(cohort 1) or 70 days later (cohort 2).
Following euthanasia, the bilateral enucleated eyes and optic
nerves were either snap frozen for Oil red O staining of fat emboli
or fixed in formaldehyde overnight and processed through a
sucrose gradient for frozen sections.
The specimens were embedded in optimal cutting temperature
(OCT) glue using and cryostat-sectioned at 14-micrometer sections
on gelatin-coated slides. Each slide contained one sample
from the control group and one experimental. Slides were stained
using Hematoxylin and Eosin (Technovit H7100/H8100, Electron
Microscopy Services, Hatfield, CT) and Oil Red O 77 (Solvent
Red 27, Sudan Red 5B; Oil Red O in 0.5% solution in propylene
glycol, Poly Scientific R&D corps, Bay Shore, NY) stains
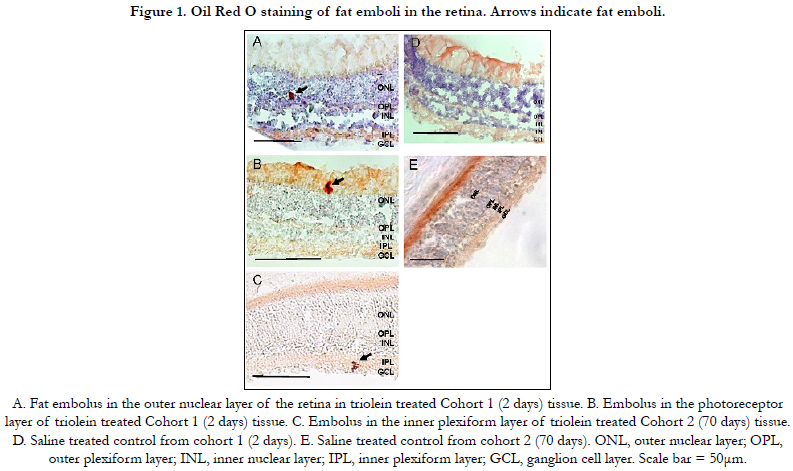
to identify fat emboli. Fat emboli were identified and imaged.
Images were taken of H&E stained and Oil Red O stains at 10x
and 20x magnification using color microscope (Axiovert 40CFL,
Zeiss, Gottingen, Germany) and Leica software (Leica Microsystems
Inc., Buffalo Grove, IL). Analysis using Image-J/FIJI software
(Image J, National Institute of Health, Bethesda, MD) was
performed to quantify the location, thickness, and size of emboli.
In statistical analysis, chi-square tests were used in intergroup
comparisons of categorical variables. In comparisons between intervention
and cohort, ANOVA was used to compare size of 86
emboli between cohorts and emboli area per retinal area between
cohorts. P values lower than 0.05 were considered statistically significant.
The analyses reported were performed using GraphPad
Prism Version 8 (GraphPad Software, San Diego, CA), Microsoft
Excel (Microsoft Corporation, Redmond, WA), and IBM SPSS
Statistics Version 25 (IBM Corp., Armonk, NY).
Results
Sixteen adult rats were distributed into two cohorts and injected
with saline or Triolein. In the acute treatment group, cohort 1, 4
rats received saline treatment and 4 received triolein treatment.
2 days later, eyes were removed and one eyecup per rat was processed
for immunohistochemical staining. In cohort 2, the long
term treatment group, 4 rats received either saline or triolein and
were sacrificed at 70 days. Samples were fixed, sectioned and subsequently
analyzed from multiple anatomically-separate regions
of the retina. Two sections were excluded from analysis due to
overstaining. Twenty-two emboli were found in samples from cohort
1 while 2 emboli were found in samples from cohort 2.
Analysis was completed with each rat as an individual unit of
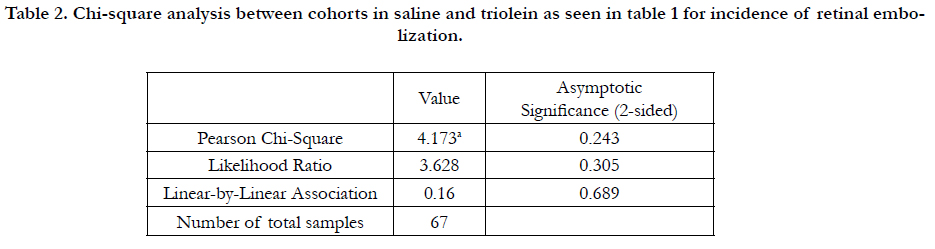
measure, as well as each slide as a unit of measure. Table 1 lists
the overall incidence of retinal embolization between all saline
treated and triolein treated rats using analysis of each slide (Pearson
chi-square: 4.173; P = 102 0.243). Triolein-treated rats in cohort
1 exhibited the greatest incidence of retinal embolization at
23.5% compared to saline-treated rats at 5.56% (Table 1). Oil red
O stains fat and will stain not only for Triolein but will also detect
endogenous lipid content in the normal eye leading to occasional
Oil Red O positivity. In just examining cohort 1 comparing saline
and triolein, several of the triolein-treated rats in cohort 1 exhibited
multiple emboli (up to 14 distributed throughout the retina).
Analysis of each rat as an individual also shows similar rates of
embolization, with the highest rate (50%) in cohort 1 triolein injected
(Chi square of 0.873 (significance 0.832)). Using Levene’s
test for equality of variances, there were significantly more emboli
in the triolein 110 treatment group of cohort 1 (P = 0.008) (Tables
1, 2).
To determine the effect of time on fat embolism, cohort 2 eyes
were examined 70 days after triolein or saline injection. Both triolein-
treated and saline-treated rats in cohort 2 had significantly
lower numbers of emboli than in cohort 1, suggesting that there
was clearance of the emboli over the 9.5-week span separating
the two groups. The strength of association between triolein administration
and embolus development based on being in either
cohort 1 and 2 was very strong based on odds ratio of 0.217 (95%
CI 0.021 to 2.191).
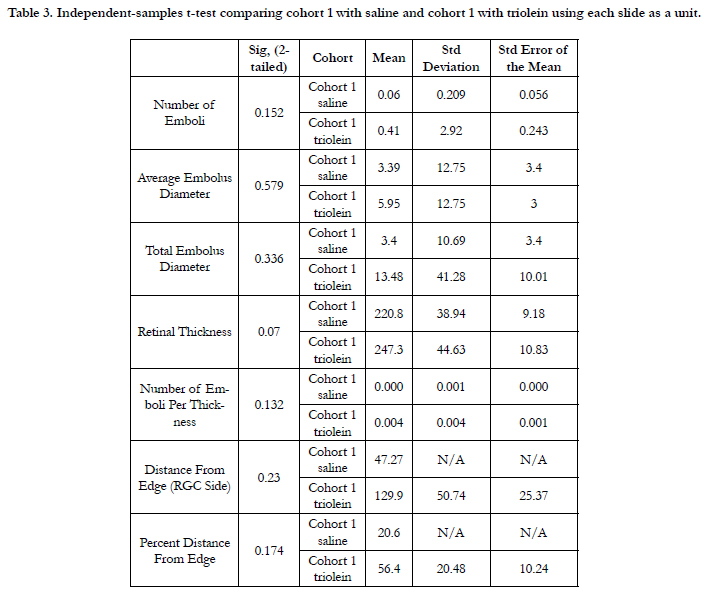
Size of emboli comparing all rats in cohort 1 and cohort 2 using
analysis by slide was not significant based on ANOVA of 0.840
(P = 0.477). Size of emboli in cohort 1 only comparing trioleintreated versus saline-treated rats showed that the average embolus
diameter was not significantly greater in the saline-treated group
(P = 0.579). Analysis of cohort 1 saline and triolein treated rats
using each rat as an individual showed that the average embolus
diameter was not significantly different (P = 0.894) (Table 3).
In the triolein treated rats, there was a wide variety in size of
emboli. In the saline treated rats, there were very few areas that
stained with Oil Red O, so the larger areas of endogenous lipid
may have appeared significant.
In both cohorts, it is expected that there would be no difference
between retinal length. This was included to facilitate the data of
emboli per length. Retinal thickness was also expected to be not
significantly different.
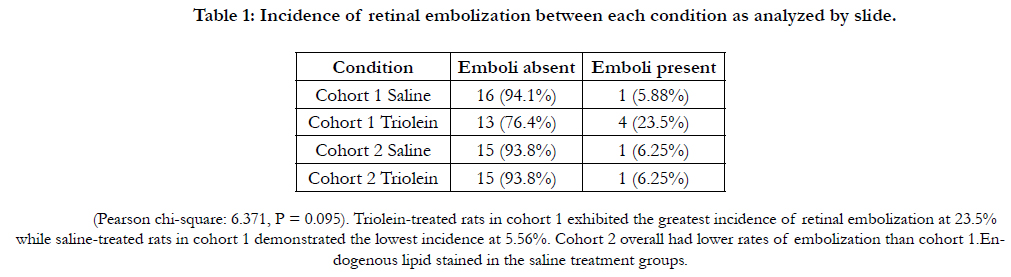
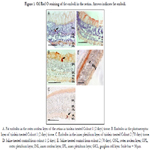
No statistically significant difference was found regarding the location
of emboli in the retina as seen in Figure 1.
Table 2. Chi-square analysis between cohorts in saline and triolein as seen in table 1 for incidence of retinal embolization.
Table 3. Independent-samples t-test comparing cohort 1 with saline and cohort 1 with triolein using each slide as a unit.
Discussion
Fat embolism syndrome (FES) occurs when fat emboli are mobilized
in the circulation, most commonly after fracture of long
bones, but also in pancreatitis, cesarean section, sickle cell bone
crisis, liposuction, and bone marrow transplant.[1, 2, 13-15] It is
thought that up to 35% of patients may have FES after traumatic
injury.[16] The mechanism behind fat release after trauma is
thought to be either from elevated intramedullary pressure following mechanical force causing direct release of fat from bone
marrow, or systemic inflammation leading to mobilization of free
fatty acids from bone marrow.[13] Fat emboli are known to cause
local inflammation and increased vessel permeability.[13] Depending
on the size of the embolism and its entry point into the
circulation, emboli can cause symptoms in tissues throughout the
body commonly including the lungs, brain, skin, and eyes. In FES,
50-60% of patients can have ocular findings on exam including
cotton wool spots and retinal hemorrhages.[2] Delayed symptom
onset is typical, with symptoms often occurring 24-48h after injury,
which has been postulated to be due to lipolysis of emboli
or delayed release of fat droplets from bone.[1, 2] Retinopathy
caused by FES typically resolves over the course of months, but
visual scotomas can persist due to changes in the retinal pigment
epithelium and optic nerve atrophy.[6]
Utilizing a rat model to induce retinal emboli allows us to characterize
these emboli and better understand the timeline of the
disease. Previous studies have examined the effect of fat emboli
on the blood-retina barrier but have not yet characterized the effects
of altering parameters of the triolein injection. This study
examined the fat emboli that occur in the retina after injection of
triolein fat at varying times. The results of this study show that the
shortest interval between injection and sacrifice had the most emboli
present. Cohort 2 which had a longer time interval (70 days)
had significantly fewer to no emboli. This shows that fat emboli
may only remain in the retina for a short period of time before
resolving but that long term damage can occur as a secondary
consequence of the fat emboli. In this study, the average embolus
diameter was 0.85μm, which is far below the average diameter of
retinal blood vessels (45μm). Large fat droplets typically get stuck
in the pulmonary vessels, but droplets that are small enough to
make it to the retina may be easily cleared.6 The cohort with the
larger dose of triolein may have had more fat emboli in their lungs
and larger vessels instead of small droplets that could make it to
the retina. Due to the clinical vision symptoms of fat embolism
to the retina, it is clear that these emboli, although small and transient,
create lasting damage.
The mechanisms behind the damage caused by these small emboli
are thought to be threefold. First, there is endothelial damage
from toxic free fatty acid release, second, there is mechanical endothelial
damage, and third, there is capillary obstruction leading
to ischemia.[2, 17] Toxic damage to endothelium is primarily due
to the hydrolysis of fat by triglyceride lipases to yield oleic acid.1
Mechanical damage to the vascular endothelium caused by these
small emboli has been shown to increase the permeability of the
blood retina barrier which could lead to long term damage in the
retina.[2] Capillary obstruction causes short term hypoxia which
can present with ischemic changes on ocular exam including cotton-
wool spots, hemorrhages, and retinal edema.[17] Emboli both
in tissue and in vasculature can cause an inflammatory reaction
which can cause local damage and long term deficiencies.
Resolution of emboli may happen through a cellular mechanism.
It has been proposed that fat droplets can be phagocytosed by
macrophages which then can stimulate an inflammatory response.
[1] Macrophages can engulf fat emboli and metabolize them into
oleic acid while also stimulating mast cells to activate the reninangiotensin-
aldosterone system.[1] It has been shown that fat emboli
in the lungs can cause pulmonary edema through this mechanism.
1 Promising animal studies in the lung have shown that the
mechanical vascular obstruction, mast cell accumulation, inflammation
and fibrosis may be ameliorated using the renin angiotensin
aldosterone system modulators including losartan, captopril,
and aliskiren,[1, 16, 18] presenting a potential target for therapy
development.[8] In our study emboli resolved spontaneously in a
short period of time. Therefore, the retinal damage may be primarily
due to the inflammatory and endothelial damage than from
ischemia. This may also mean that either preventing fat emboli in the retina or targeting early stages in the pathophysiology may
be more effective than trying to speed their destruction. Many
treatments have been used to treat the early stages of FES, but
the most consistently effective has been corticosteroids. Corticosteroids
limit free fatty acid levels, stabilize membranes, and inhibit
leukocyte aggregation.[13, 17]
In this study, significant trends in emboli size or position in the
retinal layers were not found. Previous studies have shown that
the retinal ganglion cell layer is especially susceptible to hypoxia,
but in this study, we did not find that emboli preferentially localized
to any layer.[19, 20] Sample size and the time interval may
have influenced this outcome. Neutral fat has been proven to
be the cause of FES. Therefore, the triolein model is thought to
provide a reliable model for the disease. Numerous other models
have been used to study hypoxia in the retina including a study
by Lee that used surgical occlusion of the carotid arteries.[20, 21]
Similar histopathological findings have been observed in the lungs
of patients with FES and those of rats injected with triolein.[14]
However, the fat emboli released during bone trauma also contain
the cellular component of the bone marrow.[2]
This research furthers the understanding of the timeline of the
FES disease process. Although there are other rat models for hypoxia,
this model allows a less invasive method to observe hypoxic
changes in the retina and future studies could examine the
signaling changes in the retina after this process.[21] Developing
an understanding of how hypoxia changes cellular signaling pathways
in the retina can have far reaching consequences into many
diseases processes and provide possible targets for pharmacologic
study.
Acknowledgments
Expert technical assistance from Heather Johnson, Neeru Silswal,
Suban Burale and Tianhua Lei is gratefully acknowledged. Research
reported in this publication was supported in part by the
Felix and Carmen Sabates Missouri Endowed Chair in Vision Research,
a Challenge Grant from Research to Prevent Blindness
and the Vision Research Foundation of Kansas City.
Disclosure
The authors declare that the research was conducted in the absence
of any commercial or financial relationships that could be
construed as a potential conflict of interest.
The research presented in the present publication was supported
in part by the Felix and Carmen Sabates Missouri Endowed Chair
in Vision Research, the Vision Research Foundation of Kansas
City and a departmental challenge grant by Research to Prevent
Blindness (PK) as well as by Sarah Morrison Student Research
Awards (CGO and LJR) and this support is gratefully acknowledged.
Paula Monaghan Nichols, Alan Poisner, Agostino Molteni and Peter Koulen conceived and designed the experiments; Caroline
G. Olson and Landon J. Rohowetz performed the experiments;
Caroline G. Olson, Landon J. Rohowetz, and Peter Koulen analyzed
the data and wrote the paper. All authors edited and reviewed
the paper.
References
- Poisner AM, Molteni A. Fat Embolism: What We Have Learned from Animal Models. In Embolic Diseases-Evolving Diagnostic and Management Approaches. 2019 Mar 22. IntechOpen.
- Lee JE, Jea SY, Oum BS, Kim HJ, Ohn YH.Effect of fat embolism with triolein emulsion on blood-retinal barrier. Ophthalmic Res. 2009;41(1):14-20. PubMed PMID: 18849637.
- Takakura A, Stewart PJ, Johnson RN, Cunningham ET Jr. Purtscher-like retinopathy after prostate surgery. Retin Cases Brief Rep. 2014 Fall;8(4):245- 6. PubMed PMID: 25372518.
- Lu L, Xu X, Wang Z, Ye F, Fan X. Retinal and choroidal vascular occlusion after fat injection into the temple area. Circulation. 2013 Oct 15;128(16):1797-8. PubMed PMID: 24126325.
- Scotton WJ, Kohler K, Babar J, Russell-Hermanns D, Chilvers ER. Fat embolism syndrome with Purtscher's retinopathy. Am J RespirCrit Care Med. 2013 Jan 1;187(1):106. PubMed PMID: 23281351.
- Nentwich MM, Remy M, Schaller UC. Ocular fat embolism syndrome.IntOphthalmol. 2011 Feb;31(1):15-6. PubMed PMID: 20499264.
- Colucciello M. Images in clinical medicine. Retinal arteriolar cholesterol emboli. N Engl J Med. 2008 Feb 21;358(8):826. PubMed PMID: 18287605.
- Choudhary R, Kapoor MS, Singh A, Bodakhe SH. Therapeutic targets of renin-angiotensin system in ocular disorders. J CurrOphthalmol. 2016 Oct 20;29(1):7-16. PubMed PMID: 28367520.
- de Jong EK, Geerlings MJ, den Hollander AI. Age-related macular degeneration. InGenetics and genomics of eye disease. Academic Press. 2020 Jan 1: 155-180.
- Iroku-Malize T, Kirsch S. Eye Conditions in Older Adults: Age-Related Macular Degeneration. FP Essent. 2016 Jun;445:24-8. PubMed PMID: 27348529.
- Wang HD, Zheng JH, Deng CL, Liu QY, Yang SL. Fat embolism syndromes following liposuction. Aesthetic Plast Surg. 2008 Sep;32(5):731-6. PubMed PMID: 18509699.
- İlhan Ç. Purtscher Retinopathy and Fat Embolism Syndrome. Int J Ophthalmic Res. 2019 Feb 2;5(1):299-301.
- Kwiatt ME, Seamon MJ. Fat embolism syndrome. Int J CritIllnInj Sci. 2013 Jan;3(1):64-8. PubMed PMID: 23724388.
- Ajemba O, Zia H, Lankachandra K, Singh G, Poisner A, Herndon B, et al. Fat embolism syndrome following caesarean section in an obese patient and its histopathological similarity to an animal model of FE: a case report. ClinPathol. 2015;2(3):30-5.
- Vichinsky E, Williams R, Das M, Earles AN, Lewis N, Adler A, et al. Pulmonary fat embolism: a distinct cause of severe acute chest syndrome in sickle cell anemia. Blood. 1994 Jun 1;83(11):3107-12. PubMed PMID: 8193347.
- Fletcher AN, Molteni A, Ponnapureddy R, Patel C, Pluym M, Poisner AM. The renin inhibitor aliskiren protects rat lungs from the histopathologic effects of fat embolism. J Trauma Acute Care Surg. 2017 Feb;82(2):338-344. PubMed PMID: 28107310.
- Cho KH, Ahn SJ, Cho JH, Jung C, Han MK, Park SJ, et al. The Characteristics of Retinal Emboli and its Association With Vascular Reperfusion in Retinal Artery Occlusion. Invest Ophthalmol Vis Sci. 2016 Sep 1;57(11):4589- 98. PubMed PMID: 27598864.
- Poisner A, Bass D, Fletcher A, Jain A, England JP, Davis MG, et al. Evidence for angiotensin mediation of the late histopathological effects of pulmonary fat embolism: Protection by losartan in a rat model. Exp Lung Res. 2018 Sep;44(7):361-367. PubMed PMID: 30638089.
- Kaur C, Foulds WS, Ling EA. Hypoxia-ischemia and retinal ganglion cell damage. ClinOphthalmol. 2008 Dec;2(4):879-89. PubMed PMID: 19668442.
- Linsenmeier RA, Zhang HF. Retinal oxygen: from animals to humans. ProgRetin Eye Res. 2017 May;58:115-151. PubMed PMID: 28109737.
- Lee D, Kang H, Yoon KY, Chang YY, Song HB. A mouse model of retinal hypoperfusion injury induced by unilateral common carotid artery occlusion. Exp Eye Res. 2020 Dec;201:108275. PubMed PMID: 32991884.