Treatment of Chronic Hepatitis C: An Experience Report from a Referral Center in Northeastern Brazil
Auzelivia Pastora Rego Medeiros Falcão1, Priscilla Megumi Haibara2, Fernando Henrique Destafani deSouza2, Géssika Lanzillo de Almeida Nunes2, Joyce Almeida Sá de Moraes2, AmáliaCinhtiaMeneses Rêgo3, Irami Araújo-Filho4*
1 Physician, Gastroenterologist and Hepatologist - Nucleus for Liver Studies at Hospital Universitário Onofre Lopes/Federal University of Rio Grande
do Norte/UFRN, Brazil.
2 Undergraduate Student of Medicine at UFRN - Nucleus for Liver Studies at Hospital Universitário Onofre Lopes/Federal University of Rio Grande
do Norte/UFRN, Brazil.
3 Postgraduate Program in Biotechnology at Potiguar University/UnP - Laureate International Universities. Teaching and Research Manager - School of
Health - League Against Cancer Natal - RN/Brazil. Ph.D. in Health Science.
4 Full Professor Department of Surgery, Federal University of Rio Grande do Norte. Full Professor, Department of Surgery, Potiguar University. Ph.D
in Health Science/Natal-RN, Brazil;Postgraduate Program in Biotechnology at Potiguar University/ UnP - Laureate International Universities.
*Corresponding Author
Irami Araújo-Filho - MD - Ph.D,
Postgraduate Program in Biotechnology at Potiguar University/ UnP - Laureate International Universities.
Full Professor Department of Surgery, Federal University of Rio Grande do Norte. Full Professor, Department of Surgery, Potiguar University. Ph.D in Health Science/Natal-RN, Brazil.
Tel: +55 84 98876-0206
Fax: + 55 84 3342-5079
E-mail: irami.filho@uol.com.br
Received: March 10, 2020; Accepted: July 06, 2020; Published: July 15, 2020
Citation: Auzelivia Pastora Rego Medeiros Falcão, Priscilla Megumi Haibara, Fernando Henrique Destafani deSouza, Géssika Lanzillo de Almeida Nunes, Joyce Almeida Sá de Moraes, Amália Cinhtia Meneses Rêgo, Irami Araújo-Filho. Treatment of Chronic Hepatitis C: An Experience Report from a Referral Center in Northeastern Brazil. Int J Chronic Dis Ther. 2020;6(2):102-106. doi: dx.doi.org/10.19070/2572-7613-2000021
Copyright: Irami Araújo-Filho© 2020. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Abstract
Background: Therapy for the hepatitis C virus (HCV) has undergone a revolution with the introduction of direct-acting
antivirals (DAA). DAAs achieve sustained virological response (SVR) in 90-95% of treated patients, compared to 50-70% of
those receiving dual pegylated interferon and ribavirin therapy. Although they are already available, there are few studies on
DAAs efficacy in the Brazilian population.
Objective: To evaluate the efficacy of DAAs in individuals with hepatitis C at the Liver Study Center (LSC) in Hospital Universitário
Onofre Lopes (HUOL).
Methods: Medical records of chronic HCV patients treated with DAAs from LSC were analyzed. Only those patients with a
follow-up of at least 12 weeks after the end of treatment were included.
Results: A total of 50 patients underwent treatment with DAAs at LSC. Of these, genotype 1 was present in 39 patients
(81.2%, 1a8.3%, 1b68.7%), genotype 2, in 2 patients (4.2%) and genotype 3, in 6 patients (14.5%, 3a 2%). Thirty-two were
cirrhotic (64%), and 20 were treatment-experienced (40%). The therapeutic regimens used were mainly sofosbuvir (SOF) +
simeprevir (SMV) in 23 patients (46%) and SOF + daclatasvir (DCV), in 22 (44%). SVR-12 was achieved in 92% of patients.
Four patients had virological failure: three of them were cirrhotic and treatment experienced. The other one had advanced
liver fibrosis (F3) with no previous treatment for HCV infection. No adverse events were reported during DAA treatment.
Conclusion: The experience of the LSC with DAAs showed a high rate of SVR and excellent tolerability.
2.Introduction
3.Methods
4.Results
5.Discussion
6.Conclusion
7.Acknowledgments
8.References
Keywords
Hepatitis C; Hepatic Cirrhosis; Liver Fibrosis; Liver Disease; Sustained Virologic Response; Sustained Viral Suppression; Antiviral Drugs.
Introduction
Hepatitis C virus (HCV) infection is one of the leading causes
of chronic liver disease worldwide [1]. Hepatocyte lesion induced
by HCV may lead to cirrhosis and hepatocellular carcinoma [2].
Due to the silent progression of the disease and complications
that require specialized care, hepatitis C has a significant impact
on public health [3]. In the absence of treatment, there is chronification
in 60%-85% of the cases and, on average, 20% progress
to cirrhosis over time [4]. It is estimated that 170 million people are infected, which is equivalent to 2.8% of the world population
[5-7]. In Brazil, 1.6 million people are carriers of the virus, and a
higher frequency of genotypes 1 and 3 is present, with small variations
in the prevalence ratio of these genotypes. Hepatitis C virus
exhibits high genetic diversity, characterized by regional changes
in genotype prevalence and is responsible for most deaths from
viral hepatitis in our country, representing the third leading cause
of liver transplants [8-10]. Until 2015, the treatment regimen for
HCV in Brazil was based on the combination of interferon or
interferon-pegylated with ribavirin for 48 to 72 weeks. Unfortunately,
the cure rate was only 40%-80%, and there were many
adverse effects associated [11, 12]. These unsatisfactory outcomes
led to the development of new drugs for the treatment of hepatitis
C, the so-called direct-action antivirals, representing a new era
in the history of this disease [13-15]. DAAs have brought encouraging
expectations about the potential of the cure for Hepatitis
C. Unlike the interferon regimens, this new treatment has shown
cure rates above 95% with few adverse effects, shorter duration
of therapy, and more straightforward dosing [16-18]. Monitoring
for side effects is also of little to no practical use as new DAA
regimens are generally well tolerated, with less than 1% of patients
discontinuing treatment for side effects or reporting severe
adverse events. DAAs have been initially sold at a very high
price, limiting access. Opportunities to access low-price generic
medicines are increasing [19, 20]. Since 2018, the Unified Health
System (UHS/SUS-Brazil) made available DAAs treatment for all
people with HCV infection in Brazil. Currently, the DAAs that are
part of the therapeutic arsenal offered by the SUS are sofosbuvir,
simeprevir, and daclastavir. The goal of chronic hepatitis C treatment
is to achieve the status of sustained virological response
(SVR) in post-therapy follow-up [21]. SVR is determined to be
undetectable HCV-RNA within 12 or 24 weeks after completion
of treatment. SVR is a marker of virological and clinical cure [19].
Many factors may complicate HCV medical treatment, including
the genotype of the virus, co-infections with other viruses, and
the stage of liver disease [21, 22]. Since Brazil has a considerable
extension, geographical differences in the populations studied can
also affect treatment response due to varied viral characteristics
of patients. At present, only two studies are evaluating DAAs efficacy
in Brazil, and they are restricted to the south region of the
country [23, 24]. Based on the presented scenario, this paper aims
to evaluate the efficacy of DAAs in individuals with hepatitis C
in Northeast Brazil, at the Liver Study Center (LSC) of Hospital
UniversitárioOnofre Lopes (HUOL/UFRN).
Methods
This research is a single-center retrospective observational study
using records of patients diagnosed with chronic hepatitis C who
were treated with direct-acting antiviral agents between December
2015 and February 2019. The study was carried out following
the ethical principles of the Declaration of Helsinki. The
Research Ethics Committee approved it of the Federal do Rio
Grande do Norte University (UFRN), under registration number
78858417.4.0000.5292.As a primary endpoint for our analysis, we
defined the achievement of a sustained virological response as 12
weeks after the end of antiviral treatment (SVR12). Patients that
were lost to follow up or had treatment prematurely interrupted
for any reason were excluded from this analysis. The unspecified
therapeutic regimen was also considered an exclusion criterion.
All other patients were included regardless of genotype, prior treatment, or liver fibrosis stage. Our patients were from the Liver
Study Center (LSC), the liver patient unit from Hospital Onofre
Lopes, in Natal, Rio Grande do Norte, Brazil. Data were collected
from patient’s records included demographic information (age,
gender) and clinical characteristics (disease stage, HCV genotype,
coinfection with HBV or HIV, prior transplantation, previous
therapy, side effects to DAA, and sustained virologic response).
The following DAA regimens were used for 12-24 weeks: sofosbuvir
(SOF) + simeprevir (SMV) +/- ribavirin (RBV); SOF +
daclastavir (DCV) +/- RBV. A minority of patients were treated
with alternative regimens: two with SOF + RBV, one with telaprevir
+ RBV + pegylated (PEG) interferon, and one with boceprevir
+ RBV + PEG interferon. Treatment decision was based
on the Clinical protocol and therapeutic guidelines for hepatitis
C and coinfections (PCDT), considering the availability of the
drugs in Brazil provided by the Unified Health System (UHS/
SUS-BRAZIL), the HCV virus genotype and subtype, the presence
of cirrhosis and other comorbidities and previous therapy
for HCV.
The stage of liver fibrosis was determined mainly through elastography.
Liver biopsy, APRI, and FIB4 scores were used in some
cases. Patients with clinical signs and/or echographic findings of
liver cirrhosis were considered eligible for treatment without the
need for another staging test for liver fibrosis [25].
Results were processed using standard statistical analysis. Proportions
were used for descriptive statistics. Data of 2 groups
of patients were compared using Fisher’s two-tailed test. The
p-value <0.05 was considered significant. All statistical analyses
were performed using SPSS statistics version 24 (IBM® SPSS,
Chicago, IL, USA).
Results
A total of 52 patients were treated for HCV infection in the LSC
between December 2015 and February 2019. However, two patients
were excluded due to the absence of therapeutic regimen
information. There were two patients whose HCV genotype information
was not present at the database.
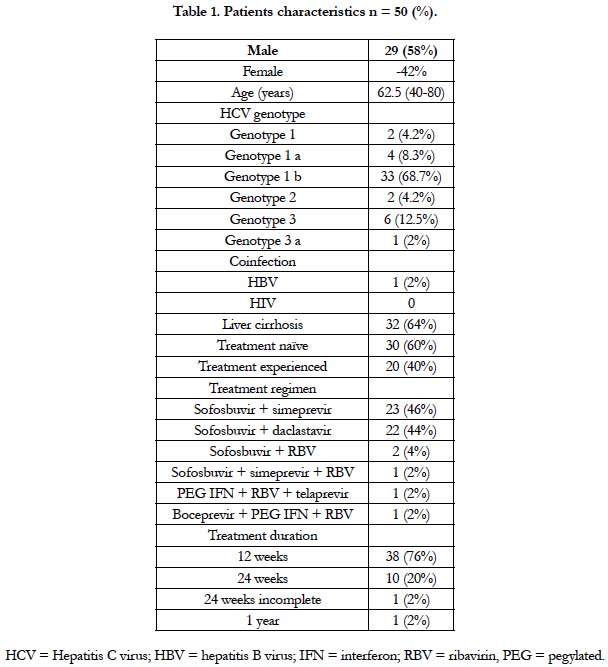
The clinical and demographic data of our patients are depicted in
Table 1. At baseline, there was a predominance of males (58%)
over females (42%), and the mean age was 62.5 years. Twenty
(40%) patients had already been treated with interferon-based
therapy in the past, and 32 (64%) were diagnosed with liver cirrhosis
before starting treatment with DAAs. One patient presented
with hepatitis B virus coinfection and diagnosis of unresectable
hepatocellular carcinoma.
In this analysis, genotype 1 was predominant (81.2%, 1a 8.3%, 1b
68.7%). Genotypes 2 and 3 represented the remaining 18.7% of
the cases. We identified that more than half of our patients had a viral load above 0,8 Mio. IU/mL.
In this retrospective study, 32 (64%) individuals had cirrhosis. Of
these, 18 (56.2%) had an F4 degree of fibrosis, and 14 (43.7%)
had clinical signs or echographic findings of cirrhosis and not
submitted for quantification of liver fibrosis. Eight patients had
advanced fibrosis (F3). Data evaluating the Child-Pugh score was
absent in most of our patients.
Almost all of our patients (96%) were treated with an interferon-
free regimen. The therapeutic regimens used were: sofosbuvir
(SOF) + simeprevir (SMV) +/- ribavirin (RBV) in 24 (48%)
patients and SOF + daclastavir (DCV) in 22 (44%) for 12 to 24
weeks. One patient was treated with boceprevir + pegylated interferon
(IFN) + RBV while the other patient was treated with telaprevir
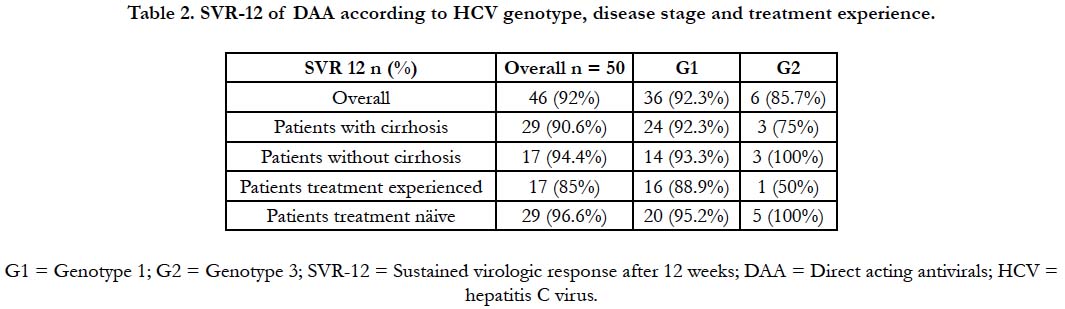
+ pegylated IFN + RBV. RVS-12 was achieved in 92% of
the subjects. Genotype 1 achieved SVR in 92,3% of the cases (1b
90.9%, 1a 100%). Genotype 2 and genotype 3 SVR was 100% and
85.7%, respectively. Treatment naïve showed SVR is 96.6%, while treatment-experienced individuals presented with 85% SVR. We
analyzed the efficacy of DAAs in this population and compared it
to the patients without cirrhosis. As stated above, 32 (64%) of our
patients were cirrhotic before starting a treatment regimen with
DAAs. The overall SVR in cirrhotics was 90.9%, whereas in noncirrhotics
SVR-12 was 94.4%. Table 2 summarizes the efficacy
of DAA therapy according to genotype, disease stage, and treatment
experience. Genotype 1 patients treated with SOF + SMP
+/- RBV achieved SVR in 91,7% of the cases, while those who
were treated with SOF + DCV +/- RBV attained SVR is 90.9%.
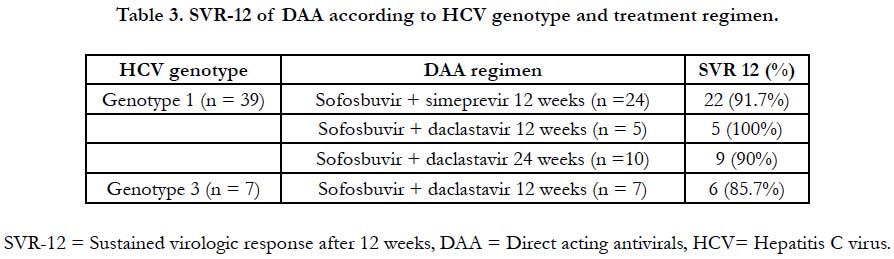
SVR, according to genotype and treatment regimen, is registered
in Table 3. Fisher’s two-tailed test comparing SOF + DCV +-
RBV and SOF + SMP +- RBV regimens in all groups of patients
(cirrhotic, treatment naïve, treatment-experienced, coinfected and
each genotype - 1a, 1b, 3a) showed p-value statistically non-significant.
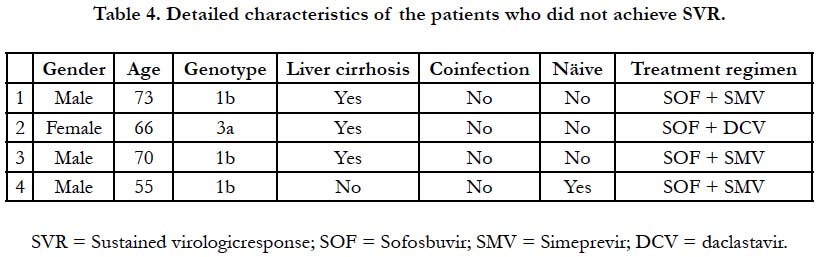
Four patients had virological failure: three of them were
cirrhotic and treatment experienced. One of the cirrhotic patients
had hepatitis B virus coinfection and unresectable hepatocellular
carcinoma. The other one had advanced liver fibrosis (F3) with no
previous treatment of HCV infection. Table 4 shows the details
of each of those patients. No adverse events were reported during
treatment with DAA.
Discussion
In this retrospective study, we evaluated the efficacy and safety of
DAA therapy in patients with HCV from an academic center in
Northeast Brazil. Similarly, to data reported in other series, there
was a predominance of males over females. Epidemiological studies
show that genotype 1 is the most common presentation of
VHC, being responsible for over half of the cases [6]. Although
genotype distribution varies depending on the region, the majority
of our patients (81.2%) belonged to genotype 1. The remaining
ones were genotype 2 and 3. There were also patients with
genotype 4 who were not included in this analysis due to a lack of
SVR-12 results. Therapeutics with SOF + DCV or SOF + SMV
for either 12 or 24 weeks demonstrated to be highly effective
and safe in patients’ treatment naïve and experienced, with excellent
tolerability and no serious adverse effects reported. Overall,
SVR-12 was achieved in 92% of our patients. SVR-12 was higher
in patients without cirrhosis (94.4%), genotype 1 (92.3%) and 2
(100%) and treatment naïve (96.6%). The results of our “reallife
experience” were similar to other studies evaluating DAAs
efficacy, including those made in the southern portion of Brazil
[23, 24, 26]. Although treatment with SOF + SMV +/- RBV
showed better response over SOF + DCV +/- RBV, there was no
statistically significant advantage of one regimen over the other
to all groups of patients specified at Table 3. Both schemes had
similar results. One randomized clinical trial also showed no statistical
significance between SOF + SMV and SOF + DCV [27].
The groups of patients’ treatment-experienced and genotype 3
with cirrhosis obtained response below 90% SVR-12. This observation
is following other reports corroborating the fact that
the population with cirrhosis or genotype 3 is the most difficult
to treat with DAAs regimens currently available [26]. However,
this data has to be interpreted with caution since the number of
our sample was limited. The presence of advanced fibrosis or cirrhosis
is known factors that affect the choice of therapy regimen
and worsens post-treatment prognosis, as well as post-treatment,
follow up the schedule [25-27]. The limitation of real-life studies
resides in the fact that it is non-randomized, allowing for selection
bias. The population in this study consisted mostly of patients
who received free therapy from Unified Health System (UHS/
SUS-Brazil).
Conclusion
In conclusion, the treatment of HCV in Northeast Brazil confirmed
DAAs high SVR rate and safety. The era of DAAs has
revolutionized HCV therapy, with the vast majority of patients
having access to treatment expected to be cured of HCV infection.
Recently approved DAA combinations herald a new paradigm
of shortened duration pan-genotypic regimens. Several factors pre-therapy still determine optimal regimens, but this may
not be required in the future as we move towards pan-genotypic
regimens. As treatments get more manageable in terms of adverse
effects, and shorter, on treatment monitoring will also diminish
for the vast majority of patients. Therefore, the introduction of
DAAs for the treatment of hepatitis C can radically change the
epidemiological picture of this disease worldwide. From the use
of these new classes of medicines, it is possible to eliminate the
infection in countries that are dedicated to responsible action to
control the epidemic, guaranteeing better results in public health
and sustainability of universal access to treatment.
Acknowledgments
The authors thank the Ph.D. in Health Sciences and Teaching
and Research Manager at League Against Cancer, Profa. Dra.
AmáliaRêgo, for her contribution and relevance to the scientific
discussion and supervision of this research, acting as an expert
consultant on the bibliographic survey, analysis, and scientific advice.
We also thank all the study components for their dedication
and effort to build a scientifically validated quality study.
References
- Lavanchy D. Evolving epidemiology of hepatitis C virus. Clinical Microbiology and Infection. 2011;17(2):107-15. PMID: 21091831.
- Lavanchy D. Chronic viral hepatitis as a public health issue in the world. Best Practice & Research Clinical Gastroenterology. 2008;22(6):991-1008. PMID: 19187863.
- Westbrook RH, Dusheiko G. Natural history of hepatitis C. Journal of Hepatology. 2014;61(1):S58-S68.
- Petruzziello A, Marigliano S, Loquercio G, Cozzolino A, Cacciapuoti C. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World journal of gastroenterology. 2016;22(34):7824-40. PMID: 27678366.
- Lavanchy D. The global burden of hepatitis C. Liver International. 2009;29(s1):74-81. PMID: 19207969.
- Messina JP, Humphreys I, Flaxman A, Brown A, Cooke GS, Pybus OG, et al. Global distribution and prevalence of hepatitis C virus genotypes. Hepatology. 2015;61(1):77-87. PMID: 25069599.
- Amaku M, Burattini MN, Coutinho FAB, Lopez LF, Mesquita F, Naveira MCM, et al. Estimating the Size of the HCV Infection Prevalence: A Modeling Approach Using the Incidence of Cases Reported to an Official Notification System. Bulletin of Mathematical Biology. 2016;78(5):970-90. PMID: 27160282.
- Campiotto S, Pinho JRR, Carrilho FJ, Da Silva LC, Souto FJD, Spinelli V, et al. Geographic distribution of hepatitis C virus genotypes in Brazil. Brazilian Journal of Medical and Biological Research. 2005;38:41-9. PMID: 15665987.
- Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis C virus choose to defer treatment: do they alter their decision with time?. Dig Dis Sci. 2007 May;52(5):1168-76. PMID: 17357838.
- Kardashian AA, Pockros PJ. Novel emerging treatments for hepatitis C infection: a fast-moving pipeline. Therapeutic Advances in Gastroenterology. 2017;10(2):277-82. PMID: 28203284.
- Butt AA, Yan P, Shaikh OS, Chung RT, Sherman KE, study tE. Sofosbuvirbased regimens in clinical practice achieve SVR rates closer to clinical trials: results from ERCHIVES. Liver International. 2016;36(5):651-8.
- Preda CM, Popescu CP, Baicus C, Voiosu TA, Manuc M, Pop CS, et al. Realworld efficacy and safety of ombitasvir, paritaprevir/r+dasabuvir+ribavirin in genotype 1b patients with hepatitis C virus cirrhosis. Liver International. 2018;38(4):602-10. PMID: 28816020.
- Aghemo A, De Francesco R. New horizons in hepatitis C antiviral therapy with direct-acting antivirals. Hepatology. 2013;58(1):428-38. PMID: 23467911.
- Pawlotsky JM. New Hepatitis C Therapies: The Toolbox, Strategies, and Challenges. Gastroenterology. 2014;146(5):1176-92. PMID: 24631495.
- Saleem A, Akhtar MF, Mushtaq MF, Saleem M, Muhammad ST, Akhtar B, et al. Current trends in the treatment of hepatitis C: interventions to avoid adverse effects and increase effectiveness of anti-HCV drugs. EXCLI journal. 2016;15:578-88. PMID: 28096788.
- Pawlotsky J-M, Negro F, Aghemo A, Berenguer M, Dalgard O, Dusheiko G, et al. EASL Recommendations on Treatment of Hepatitis C 2018. Journal of Hepatology. 2018;69(2):461-511. PMID: 29650333.
- Khokhar OS, Lewis JH. Reasons why patients infected with chronic hepatitis Cvirus choose to defer treatment: do they alter their decision with time?. Dig Dis Sci. 2007 May;52(5):1168-76. PMID: 17357838.
- Panel AIHG. Hepatitis C guidance: AASLD-IDSA recommendations for testing, managing, and treating adults infected with hepatitis C virus. Hepatology. 2015;62(3):932-54. PMID: 26111063.
- Aghemo A, Piroth L, Bhagani S. What do clinicians need to watch for with direct-acting antiviral therapy?. Journal of the International AIDS Society. 2018;21(S2):e25076. PMID: 29633552.
- Sette-Jr H, Cheinquer H, Wolff FH, de Araujo A, Coelho-Borges S, Soares SRP, et al. Treatment of Chronic HCV Infection with the New Direct Acting Antivirals (DAA): First Report of a RealWorld Experience in Southern Brazil. Annals of Hepatology. 2017;16(5):727-33. PMID: 28809742.
- Ferreira VL, Borba HHL, Wiens A, Pedroso MLA, RadunzVFdC, Ivantes CAP, et al. Effectiveness and tolerability of direct-acting antivirals for chronic hepatitis C patients in a Southern state of Brazil. The Brazilian Journal of Infectious Diseases. 2018;22(3):186-92. PMID: 29752891.
- European Association for the Study of the Liver.EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. Journal of Hepatology. 2015;63(1):237-64. PMID: 25911335.
- Ioannou GN, Beste LA, Chang MF, Green PK, Lowy E, Tsui JI, et al. Effectiveness of Sofosbuvir, Ledipasvir/Sofosbuvir, or Paritaprevir/Ritonavir/ Ombitasvir and Dasabuvir Regimens for Treatment of Patients With Hepatitis C in the Veterans Affairs National Health Care System. Gastroenterology. 2016;151(3):457-71.e5. PMID: 27267053.
- Pott H, Bricks G, Grandi G, Senise JF, Castelo A. Sofosbuvir in combination with daclatasvir or simeprevir for 12 weeks in non-cirrhotic individuals chronically infected with hepatitis virus genotype 1: a randomized clinical trial. Clin Microbiol Infect. 2019; 25(3): 365-371. PMID: 29906601.
- Nelson DR, Cooper JN, Lalezari JP, Lawitz E, Pockros PJ, Gitlin N, et al. All-oral 12-week treatment with daclatasvir plus sofosbuvir in patients with hepatitis C virus genotype 3 infection: ALLY-3 phase III study. Hepatology. 2015;61(4):1127-35. PMID: 25614962.
- Leroy V, Angus P, Bronowicki J-P, Dore GJ, Hezode C, Pianko S, et al. Daclatasvir, sofosbuvir, and ribavirin for hepatitis C virus genotype 3 and advanced liver disease: A randomized phase III study (ALLY-3+). Hepatology. 2016;63(5):1430-41. PMID: 26822022.
- Welzel TM, Petersen J, Herzer K, Ferenci P, Gschwantler M, Wedemeyer H, et al. Daclatasvir plus sofosbuvir, with or without ribavirin, achieved high sustained virological response rates in patients with HCV infection and advanced liver disease in a real-world cohort. Gut. 2016;65(11):1861-1870. PMID: 27605539.